We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox 39
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
=='''Papain'''== | =='''Papain'''== | ||
| - | < | + | <StructureSection load='9pap' size='300' side='right' caption='Structure (PDB entry [[9pap]])' scene=''> |
== Introduction == | == Introduction == | ||
Papain (9PAP), also known as papaya proteinase I, is an enzyme found in unripe papaya fruit. A cysteine protease, it has been used to break down tough muscle fibers, and hence is often found in powdered meat tenderizers. It has also been used in cell isolation procedures because it is very efficient and not very destructive. It is collected from the fruit by scoring its skin and allowing the "sap" to seem out. The sap is then dried and purified. | Papain (9PAP), also known as papaya proteinase I, is an enzyme found in unripe papaya fruit. A cysteine protease, it has been used to break down tough muscle fibers, and hence is often found in powdered meat tenderizers. It has also been used in cell isolation procedures because it is very efficient and not very destructive. It is collected from the fruit by scoring its skin and allowing the "sap" to seem out. The sap is then dried and purified. | ||
| + | |||
| + | The 3D model of papain shown to the right is "decorated" with solvent methanol molecules. The following Jmol representations of papain will not show these solvent molecules. | ||
== Function == | == Function == | ||
| Line 30: | Line 32: | ||
== Composition of Papain == | == Composition of Papain == | ||
| - | Proteins only consist of certain elements: carbon, hydrogen, nitrogen, oxygen, and sulfur. Enzymes' primary structures allow them to fold optimally and interact with their substrates maximally in order to efficiently catalyze biological reactions. The <scene name='Sandbox_39/ | + | Proteins only consist of certain elements: carbon, hydrogen, nitrogen, oxygen, and sulfur. Enzymes' primary structures allow them to fold optimally and interact with their substrates maximally in order to efficiently catalyze biological reactions. The <scene name='Sandbox_39/Elemental/2'>elemental composition</scene> of papain shows carbon atoms outlined in grey, oxygen atoms in red, nitrogen atoms in blue, and sulfur atoms in yellow. |
| - | In addition, the entirety of the secondary structure of papain can be traced from the <scene name='Sandbox_39/ | + | In addition, the entirety of the secondary structure of papain can be traced from the <scene name='Sandbox_39/Elemental/3'>N- to C-terminus.</scene> As shown to the left, the red end begins the protein at the |
N-terminus, and can be traced through the colors of the rainbow to the purple end at the C-terminus. | N-terminus, and can be traced through the colors of the rainbow to the purple end at the C-terminus. | ||
| Line 37: | Line 39: | ||
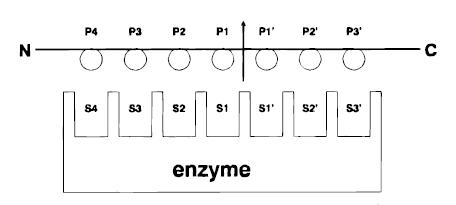
Papain has a broad specificity for protein substrates. The active site consists of seven subsites (S1-S4 and S1’-S3’) that can each accommodate one amino acid residue of a protein substrate (P1-P4 and P1’-P3’). | Papain has a broad specificity for protein substrates. The active site consists of seven subsites (S1-S4 and S1’-S3’) that can each accommodate one amino acid residue of a protein substrate (P1-P4 and P1’-P3’). | ||
[[Image:Subsites.jpg|right]] | [[Image:Subsites.jpg|right]] | ||
| - | Specificity is controlled, however, by the <scene name='Sandbox_39/Catalytic_triad/ | + | Specificity is controlled, however, by the <scene name='Sandbox_39/Catalytic_triad/2'>catalytic triad</scene>, a hydrophobic pocket that accommodates the side chains of the protein substrate. This triad consists of a histidine, asparagine, and a cysteine, after which the protein is categorized as a cysteine protease. Papain exhibits specific substrate preferences for hydrophobic or aromatic residues. |
== Secondary Structure == | == Secondary Structure == | ||
| - | Papain's primary structure causes it to fold into different motifs that make up its secondary structure. These motifs include <scene name='Sandbox_39/Alpha_helices/ | + | Papain's primary structure causes it to fold into different motifs that make up its secondary structure. These motifs include <scene name='Sandbox_39/Alpha_helices/2'>alpha helices</scene>, shown in green, and <scene name='Sandbox_39/Beta_pleated_sheets/2'>beta pleated sheets</scene>, shown in orange. As shown to the left, papain has 7 alpha helices and 8 beta pleated sheets. All other motifs are nonrandom, structural units, mostly simply turns. |
== Polarity and Hydrophobicity == | == Polarity and Hydrophobicity == | ||
| - | Papain contains | + | Papain contains many hydrophobic, or "water-hating" regions, and hydrophillic, or "water-loving" regions. The hydrophobic effect, or the tendency of nonpolar substances to aggregate in aqueous solution and exclude water molecules, allows proteins to fold the way they do, exposing hydrophilic residues on their outer surface while sequestering hydrophobic residues in their center. As shown above, all of the <scene name='Sandbox_39/Hydrophobic/1'>hydrophobic residues</scene> are found in the center of the folded protein, shown at the left in pink. |
== Disulfide Bonds == | == Disulfide Bonds == | ||
Revision as of 19:39, 13 November 2011
| Please do NOT make changes to this Sandbox. Sandboxes 30-60 are reserved for use by Biochemistry 410 & 412 at Messiah College taught by Dr. Hannah Tims during Fall 2012 and Spring 2013. |
Papain
| |||||||||||