We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Journal:PLoS ONE:2
From Proteopedia
(Difference between revisions)

| Line 9: | Line 9: | ||
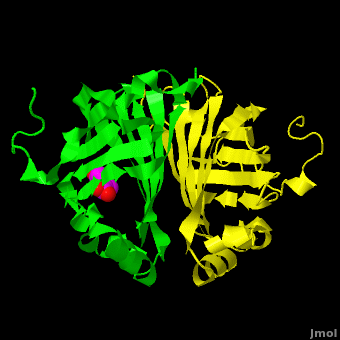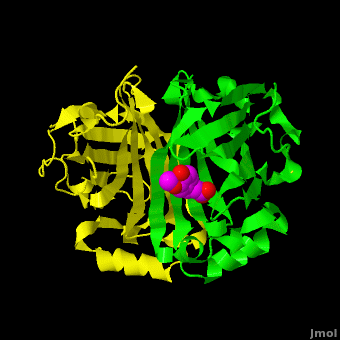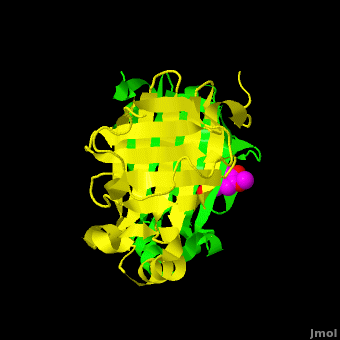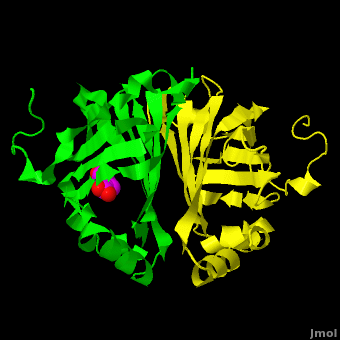
Gu ''et al.'' reported the crystal structure of ferulic acid decarboxylase (FADase) from ''Enterobacter sp.'' Px6-4. The enzyme catalyzes the decarboxylation of ferulic acid, to give 4-vinylguaiacol, a potential precursor for vanillin. The crystals obtained contained two enzyme molecules in the asymmetric unit. One structure was of the “native” conformation, with no substrate bound in the active site of either monomer. <scene name='Journal:PLoS_ONE:2/Cv/4'>A second structure was obtained by soaking the crystals in a sodium ferulate solution.</scene> In this crystal structure <scene name='Journal:PLoS_ONE:2/Cv/6'>one of the enzyme monomers (chain A)</scene> appears to have undergone significant structural changes, with electron density appearing for a <scene name='Journal:PLoS_ONE:2/Cv/7'>small molecule bound in the active site</scene>. The <scene name='Journal:PLoS_ONE:2/Cv/4'>second monomer showed no evidence of change</scene>, and no unusual electron density was observed in the active site. The authors modeled an unreacted ferulate molecule into the density in the active site of the first monomer, and attributed the lack of binding in the second monomer to close crystal contacts, which limit the necessary movements required to allow binding. This latter structure was deposited in the PDB with accession code [[3nx2]]. | Gu ''et al.'' reported the crystal structure of ferulic acid decarboxylase (FADase) from ''Enterobacter sp.'' Px6-4. The enzyme catalyzes the decarboxylation of ferulic acid, to give 4-vinylguaiacol, a potential precursor for vanillin. The crystals obtained contained two enzyme molecules in the asymmetric unit. One structure was of the “native” conformation, with no substrate bound in the active site of either monomer. <scene name='Journal:PLoS_ONE:2/Cv/4'>A second structure was obtained by soaking the crystals in a sodium ferulate solution.</scene> In this crystal structure <scene name='Journal:PLoS_ONE:2/Cv/6'>one of the enzyme monomers (chain A)</scene> appears to have undergone significant structural changes, with electron density appearing for a <scene name='Journal:PLoS_ONE:2/Cv/7'>small molecule bound in the active site</scene>. The <scene name='Journal:PLoS_ONE:2/Cv/4'>second monomer showed no evidence of change</scene>, and no unusual electron density was observed in the active site. The authors modeled an unreacted ferulate molecule into the density in the active site of the first monomer, and attributed the lack of binding in the second monomer to close crystal contacts, which limit the necessary movements required to allow binding. This latter structure was deposited in the PDB with accession code [[3nx2]]. | ||
| - | The editors of the journal were contacted by a reader, who raised concerns about the accuracy of the assignment of the density in the <scene name='Journal:PLoS_ONE:2/Cv/9'>active site of chain A</scene> of [[3nx2]]. The reader suggested that the density was more reminiscent of a <scene name='Journal:PLoS_ONE:2/Cv/10'>HEPES molecule</scene> (used in the crystallization buffer) based on the shape of the density, and the fact that the stereochemistry in the region of the double bond was distorted. In response to these concerns the Editors contacted one of us (JLS), asking for a critical review of the claims of the authors regarding 3NX2. The results of this review are detailed below. | + | The editors of the journal were contacted by a reader, who raised concerns about the accuracy of the assignment of the density in the <scene name='Journal:PLoS_ONE:2/Cv/9'>active site of chain A</scene> of [[3nx2]]. The reader suggested that the density was more reminiscent of a <scene name='Journal:PLoS_ONE:2/Cv/10'>HEPES molecule</scene> (used in the crystallization buffer) based on the shape of the density, and the fact that the stereochemistry in the region of the double bond was distorted. <font color='red'><b>O atoms are colored in red</b></font>, <font color='blue'><b>N atoms are in blue</b></font>, In response to these concerns the Editors contacted one of us (JLS), asking for a critical review of the claims of the authors regarding 3NX2. The results of this review are detailed below. |
The structure of 3nx2 was downloaded from the PDB as well as the structure factors. The software suite PHENIX[2] was used to run simulated annealing on the structure, with the ligand removed. This produced a simulated annealing Fo - Fc omit map, which should have all structural bias caused by the original ligand removed. A HEPES molecule was fitted into this density using COOT[3], and refined in PHENIX (R = 19.4%, Rfree = 24.4%). Although the R and Rfree are slightly higher than for 3nx2 (R =18.9%, Rfree = 23.6%), this is to be expected, since the geometry of the model refined in PHENIX (rms bonds 0.007, angles 1.12) was much tighter than the one obtained from REFMAC[4] (rms bonds 0.019, angles 1.81). In Figure 2, we superimpose the omit map with the two refined structures: on the left, 3NX2, with magenta carbon atoms, and on the right, with cyan carbon atoms, the PHENIX-refined enzyme-HEPES complex. | The structure of 3nx2 was downloaded from the PDB as well as the structure factors. The software suite PHENIX[2] was used to run simulated annealing on the structure, with the ligand removed. This produced a simulated annealing Fo - Fc omit map, which should have all structural bias caused by the original ligand removed. A HEPES molecule was fitted into this density using COOT[3], and refined in PHENIX (R = 19.4%, Rfree = 24.4%). Although the R and Rfree are slightly higher than for 3nx2 (R =18.9%, Rfree = 23.6%), this is to be expected, since the geometry of the model refined in PHENIX (rms bonds 0.007, angles 1.12) was much tighter than the one obtained from REFMAC[4] (rms bonds 0.019, angles 1.81). In Figure 2, we superimpose the omit map with the two refined structures: on the left, 3NX2, with magenta carbon atoms, and on the right, with cyan carbon atoms, the PHENIX-refined enzyme-HEPES complex. | ||
Revision as of 13:15, 24 November 2011
| |||||||||||
- ↑ Gu W, Yang J, Lou Z, Liang L, Sun Y, Huang J, Li X, Cao Y, Meng Z, Zhang KQ. Structural Basis of Enzymatic Activity for the Ferulic Acid Decarboxylase (FADase) from Enterobacter sp. Px6-4. PLoS One. 2011 Jan 21;6(1):e16262. PMID:21283705 doi:10.1371/journal.pone.0016262
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.