ToxT
From Proteopedia
| Line 8: | Line 8: | ||
<b>ToxT</b> is a molecule at the end of a transcriptional cascade that autoregulates the transcription of the primary virulence factors of <i>Vibrio cholerae</i>[http://http://en.wikipedia.org/wiki/Vibrio_cholerae] and itself. These two factors, cholera toxin (CT)[http://http://en.wikipedia.org/wiki/Cholera_toxin] and the toxin co-regulated pilus (TCP), are instrumental in causing the disease <b>cholera</b>[http://http://en.wikipedia.org/wiki/Cholera]. This is an intestinal infection resulting in massive water loss in the affected individual, causing extreme dehydration.[http://http://en.wikipedia.org/wiki/Cholera] | <b>ToxT</b> is a molecule at the end of a transcriptional cascade that autoregulates the transcription of the primary virulence factors of <i>Vibrio cholerae</i>[http://http://en.wikipedia.org/wiki/Vibrio_cholerae] and itself. These two factors, cholera toxin (CT)[http://http://en.wikipedia.org/wiki/Cholera_toxin] and the toxin co-regulated pilus (TCP), are instrumental in causing the disease <b>cholera</b>[http://http://en.wikipedia.org/wiki/Cholera]. This is an intestinal infection resulting in massive water loss in the affected individual, causing extreme dehydration.[http://http://en.wikipedia.org/wiki/Cholera] | ||
==Structural Features== | ==Structural Features== | ||
| - | ToxT belongs to a family of transcriptional regulators known as AraC.<ref name="structure">PMID: 20133655</ref> The AraC family is defined by a 100 amino acid region of sequence similarity that forms a DNA-binding domain with two helix-turn-helix motifs. <ref name="arac">PMID: 11282467</ref> | + | ToxT belongs to a family of transcriptional regulators known as AraC.<ref name="structure">PMID: 20133655</ref> The AraC family is defined by a 100 amino acid region of sequence similarity that forms a DNA-binding domain with two helix-turn-helix motifs. <ref name="arac">PMID: 11282467</ref> The two HTH regions are linked by another alpha helix, which is very polar. |
<br/> | <br/> | ||
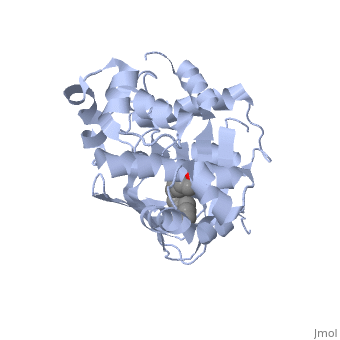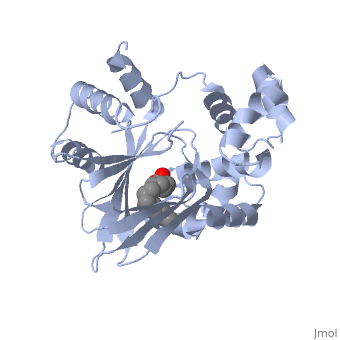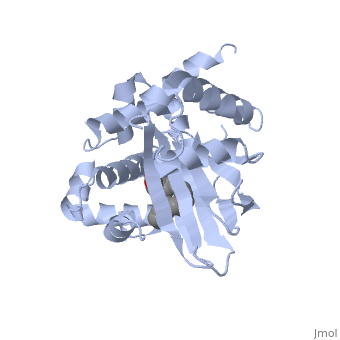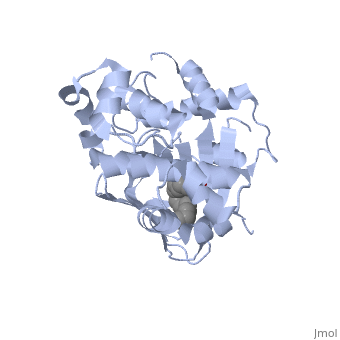
| - | A nine-stranded beta sheet sandwich<!--insert scene here!--> contains a binding pocket that contains cis-palmitoleate,<!--insert scene here!--> <ref name="structure">PMID: 20133655</ref> which appears to have a negative effect on virulence when present in vitro. This unsaturated fatty acid, like other UFAs,[http://http://en.wikipedia.org/wiki/Fatty_acid#Unsaturated_fatty_acids] tend to inhibit genes under the control of ToxT. | + | A nine-stranded beta sheet sandwich<!--insert scene here!--> contains a binding pocket that contains a ligand: cis-palmitoleate,<!--insert scene here!--> <ref name="structure">PMID: 20133655</ref> which appears to have a negative effect on virulence when present in vitro. This unsaturated fatty acid, like other UFAs,[http://http://en.wikipedia.org/wiki/Fatty_acid#Unsaturated_fatty_acids] tend to inhibit genes under the control of ToxT. |
| + | Specifically, the cis-palmitoleate appears to bind directly to ToxT, change its conformation, and thus lower the ability to bind DNA and form dimers.<ref name="structure">PMID: 20133655</ref> | ||
| + | </br> | ||
| + | Though the structure shown is a monomer with two overall domains (N-terminal and C-terminal), ToxT tends to form a dimer. The preferred state of ToxT varies between promoters, but binding to the <i>ctx</i> promoter to generate cholera toxin appears to be possible only in the dimer form. <ref name="virstatin">PMID: 1892951</ref> | ||
==References== | ==References== | ||
<references/> | <references/> | ||
Revision as of 23:58, 26 November 2011
|
Introduction
ToxT is a molecule at the end of a transcriptional cascade that autoregulates the transcription of the primary virulence factors of Vibrio cholerae[1] and itself. These two factors, cholera toxin (CT)[2] and the toxin co-regulated pilus (TCP), are instrumental in causing the disease cholera[3]. This is an intestinal infection resulting in massive water loss in the affected individual, causing extreme dehydration.[4]
Structural Features
ToxT belongs to a family of transcriptional regulators known as AraC.[1] The AraC family is defined by a 100 amino acid region of sequence similarity that forms a DNA-binding domain with two helix-turn-helix motifs. [2] The two HTH regions are linked by another alpha helix, which is very polar.
A nine-stranded beta sheet sandwich contains a binding pocket that contains a ligand: cis-palmitoleate, [1] which appears to have a negative effect on virulence when present in vitro. This unsaturated fatty acid, like other UFAs,[5] tend to inhibit genes under the control of ToxT.
Specifically, the cis-palmitoleate appears to bind directly to ToxT, change its conformation, and thus lower the ability to bind DNA and form dimers.[1]
</br>
Though the structure shown is a monomer with two overall domains (N-terminal and C-terminal), ToxT tends to form a dimer. The preferred state of ToxT varies between promoters, but binding to the ctx promoter to generate cholera toxin appears to be possible only in the dimer form. [3]
References
- ↑ 1.0 1.1 1.2 Lowden MJ, Skorupski K, Pellegrini M, Chiorazzo MG, Taylor RK, Kull FJ. Structure of Vibrio cholerae ToxT reveals a mechanism for fatty acid regulation of virulence genes. Proc Natl Acad Sci U S A. 2010 Feb 16;107(7):2860-5. Epub 2010 Feb 1. PMID:20133655
- ↑ Martin RG, Rosner JL. The AraC transcriptional activators. Curr Opin Microbiol. 2001 Apr;4(2):132-7. PMID:11282467
- ↑ Mzareulov KD. [The effect of amino acid substitutions on the 3-dimensional structure of fragment 1--16 of the oncoprotein p21 ras]. Nauchnye Doki Vyss Shkoly Biol Nauki. 1991;(5):38-45. PMID:1892951
Proteopedia Page Contributors and Editors (what is this?)
Ingrid Youngworth, Yang Yang, Michal Harel, Alexander Berchansky, Jaime Prilusky