This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
ToxT
From Proteopedia
| Line 13: | Line 13: | ||
Specifically, the cis-palmitoleate appears to bind directly to ToxT, change its conformation, and thus lower the ability to bind DNA and form dimers.<ref name="structure">PMID: 20133655</ref> | Specifically, the cis-palmitoleate appears to bind directly to ToxT, change its conformation, and thus lower the ability to bind DNA and form dimers.<ref name="structure">PMID: 20133655</ref> | ||
</br> | </br> | ||
| - | Though the structure shown is a monomer with two overall domains (N-terminal and C-terminal), ToxT tends to form a dimer. The preferred state of ToxT varies between promoters, but binding to the <i>ctx</i> promoter to generate cholera toxin appears to be possible only in the dimer form. <ref name="virstatin">PMID: | + | Though the structure shown is a monomer with two overall domains (N-terminal and C-terminal), ToxT tends to form a dimer. The preferred state of ToxT varies between promoters, but binding to the <i>ctx</i> promoter to generate cholera toxin appears to be possible only in the dimer form. <ref name="virstatin">PMID:17283330</ref> |
==References== | ==References== | ||
<references/> | <references/> | ||
Revision as of 23:59, 26 November 2011
|
Introduction
ToxT is a molecule at the end of a transcriptional cascade that autoregulates the transcription of the primary virulence factors of Vibrio cholerae[1] and itself. These two factors, cholera toxin (CT)[2] and the toxin co-regulated pilus (TCP), are instrumental in causing the disease cholera[3]. This is an intestinal infection resulting in massive water loss in the affected individual, causing extreme dehydration.[4]
Structural Features
ToxT belongs to a family of transcriptional regulators known as AraC.[1] The AraC family is defined by a 100 amino acid region of sequence similarity that forms a DNA-binding domain with two helix-turn-helix motifs. [2] The two HTH regions are linked by another alpha helix, which is very polar.
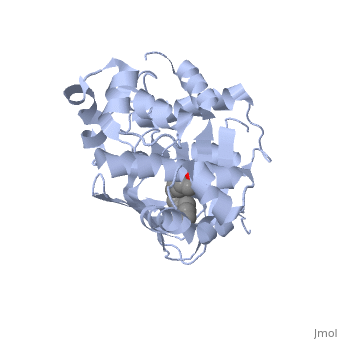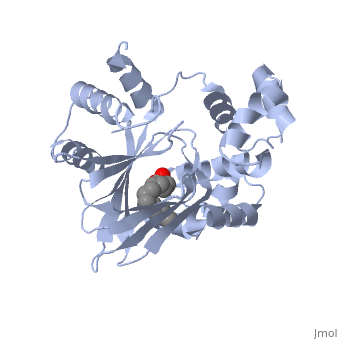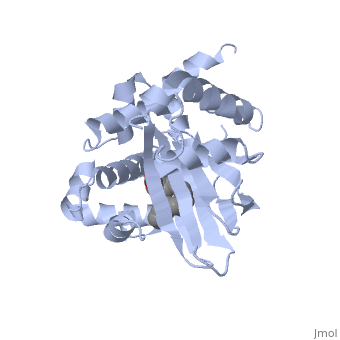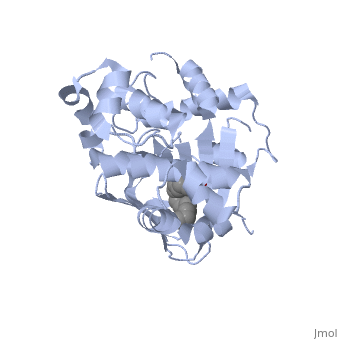
A nine-stranded beta sheet sandwich contains a binding pocket that contains a ligand: cis-palmitoleate, [1] which appears to have a negative effect on virulence when present in vitro. This unsaturated fatty acid, like other UFAs,[5] tend to inhibit genes under the control of ToxT.
Specifically, the cis-palmitoleate appears to bind directly to ToxT, change its conformation, and thus lower the ability to bind DNA and form dimers.[1]
</br>
Though the structure shown is a monomer with two overall domains (N-terminal and C-terminal), ToxT tends to form a dimer. The preferred state of ToxT varies between promoters, but binding to the ctx promoter to generate cholera toxin appears to be possible only in the dimer form. [3]
References
- ↑ 1.0 1.1 1.2 Lowden MJ, Skorupski K, Pellegrini M, Chiorazzo MG, Taylor RK, Kull FJ. Structure of Vibrio cholerae ToxT reveals a mechanism for fatty acid regulation of virulence genes. Proc Natl Acad Sci U S A. 2010 Feb 16;107(7):2860-5. Epub 2010 Feb 1. PMID:20133655
- ↑ Martin RG, Rosner JL. The AraC transcriptional activators. Curr Opin Microbiol. 2001 Apr;4(2):132-7. PMID:11282467
- ↑ Shakhnovich EA, Hung DT, Pierson E, Lee K, Mekalanos JJ. Virstatin inhibits dimerization of the transcriptional activator ToxT. Proc Natl Acad Sci U S A. 2007 Feb 13;104(7):2372-7. Epub 2007 Feb 5. PMID:17283330 doi:10.1073/pnas.0611643104
Proteopedia Page Contributors and Editors (what is this?)
Ingrid Youngworth, Yang Yang, Michal Harel, Alexander Berchansky, Jaime Prilusky