User:Brian Hernandez/DOPA Decarboxylase
From Proteopedia
(→'''Inhibitor Binding''') |
|||
| Line 11: | Line 11: | ||
==Function== | ==Function== | ||
==='''The Active Site'''=== | ==='''The Active Site'''=== | ||
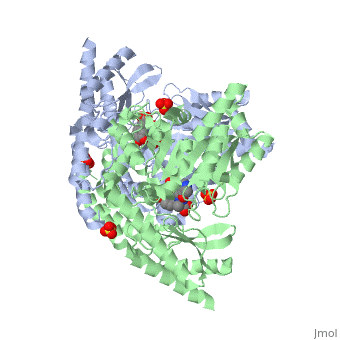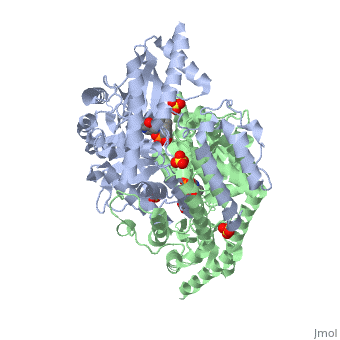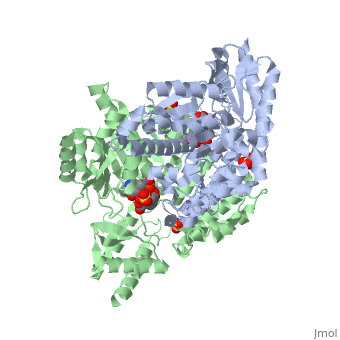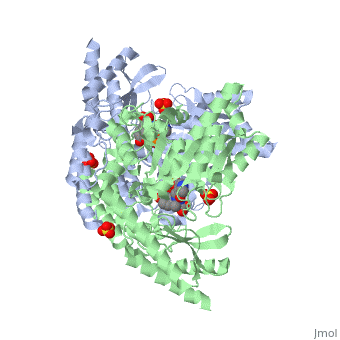
| - | DDC's active site is located in a cleft between the two monomer subunits, but is composed mainly of residues from one monomer.The active site is composed of several key residues, including Lys-303, Asp-271, His-192, Thr-82, Ile-101, and Phe-103. In the ligand free form, PLP binds to Lys 303 via a Schiff base linkage. A salt bridge forms between the carboxylate group of Asp 271 and the protonated pyridine nitrogen of PLP yielding a strong electron sink capable of stabilizing the carbanionic intermediates. The only two active site residues from the adjacent monomer, Ile-101 and Phe-103, are part of the substrate binding pocket. | + | DDC's active site is located in a <scene name='DOPA_decarboxylase/Dimer_interface/2'>cleft</scene> between the two monomer subunits, but is composed mainly of residues from one monomer.The <scene name='DOPA_decarboxylase/Active_site/1'>active site</scene> is composed of several key residues, including Lys-303, Asp-271, His-192, Thr-82, Ile-101, and Phe-103. In the ligand free form, PLP binds to Lys 303 via a Schiff base linkage. A [http://en.wikipedia.org/wiki/Salt_bridge_(protein) salt bridge] forms between the carboxylate group of Asp 271 and the protonated pyridine nitrogen of PLP yielding a strong electron sink capable of stabilizing the carbanionic intermediates. The only two active site residues from the adjacent monomer, Ile-101 and Phe-103, are part of the substrate binding pocket. |
| + | [[image:plp bound.png|thumb|center|400px|'''Schiff base linkage of PLP to Lys303 in the active site''']] | ||
==='''Flexible Loop'''=== | ==='''Flexible Loop'''=== | ||
| Line 20: | Line 21: | ||
==DDC and Parkinson's Disease== | ==DDC and Parkinson's Disease== | ||
==='''Treatment'''=== | ==='''Treatment'''=== | ||
| - | Parkinson's disease, a neurological disorder, can be characterized by tremor, bradykinesia, rigidity, and postural instability. With it's possible relation to degenerative dopamine-producing cells in the brain, administration of L-DOPA can increase the amount of synthesized dopamine in the nerve cell; direct treatment with dopamine is not sufficient as dopamine itself cannot pass the blood-brain barrier. However, only a small percentage of the dose actually reaches the nervous system, with the remaining majority being rapidly converted to dopamine in the blood stream. This dopamine-rich blood causes side effects of nausea, daytime sleepiness, orthostatic hypotension, involuntary movements, decreased appetite, insomnia, and cramping. Addition of a DDC inhibitor would block peripheral conversion to dopamine and allow a greater percentage of L-DOPA to reach the brain, causing an increase in brain dopamine levels, and diminishing the side effects of dopamine-rich blood. | + | Parkinson's disease, a neurological disorder, can be characterized by [http://en.wikipedia.org/wiki/Tremor tremor], [http://en.wikipedia.org/wiki/Bradykinesia#Bradykinesia bradykinesia], rigidity, and postural instability. With it's possible relation to degenerative dopamine-producing cells in the brain, administration of L-DOPA can increase the amount of synthesized dopamine in the nerve cell; direct treatment with dopamine is not sufficient as dopamine itself cannot pass the blood-brain barrier. However, only a small percentage of the dose actually reaches the nervous system, with the remaining majority being rapidly converted to dopamine in the blood stream. This dopamine-rich blood causes side effects of nausea, daytime sleepiness, orthostatic hypotension, involuntary movements, decreased appetite, insomnia, and cramping. Addition of a DDC inhibitor would block peripheral conversion to dopamine and allow a greater percentage of L-DOPA to reach the brain, causing an increase in brain dopamine levels, and diminishing the side effects of dopamine-rich blood. |
==='''Inhibitor Binding'''=== | ==='''Inhibitor Binding'''=== | ||
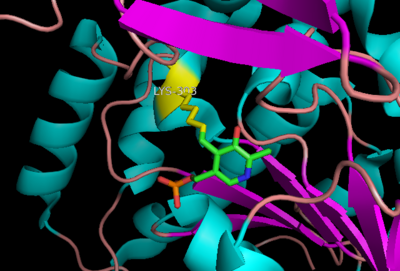
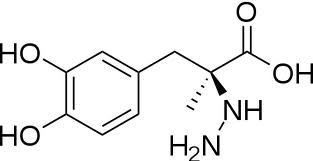
The inhibitor '''carbiDOPA''' binds to the enzyme by forming a hydrazone linkage with the PLP cofactor through its hydrazine moiety <ref name=Burkhard>PMID: 11685243 </ref>. | The inhibitor '''carbiDOPA''' binds to the enzyme by forming a hydrazone linkage with the PLP cofactor through its hydrazine moiety <ref name=Burkhard>PMID: 11685243 </ref>. | ||
[[Image:carbidopa.jpeg|thumb|right|400px|'''carbiDOPA''']] The catechol ring buries itself deep within the active site cleft, with the 4' hydroxyl of the catechol ring hydrogen bonded to the hydroxyl group of Thr 82. Additionally, the highly conserved His 192 residue forms a hydrogen bond to the carboxylate group of carbiDOPA (with the carboxylate group of L-DOPA being even closer in binding range). Thus, His 192 is most likely a key residue in the enzyme's catalytic activity because a H192A mutation can cause complete loss of activity. | [[Image:carbidopa.jpeg|thumb|right|400px|'''carbiDOPA''']] The catechol ring buries itself deep within the active site cleft, with the 4' hydroxyl of the catechol ring hydrogen bonded to the hydroxyl group of Thr 82. Additionally, the highly conserved His 192 residue forms a hydrogen bond to the carboxylate group of carbiDOPA (with the carboxylate group of L-DOPA being even closer in binding range). Thus, His 192 is most likely a key residue in the enzyme's catalytic activity because a H192A mutation can cause complete loss of activity. | ||
Revision as of 05:57, 29 November 2011
| |||||||||||
DDC and Parkinson's Disease
Treatment
Parkinson's disease, a neurological disorder, can be characterized by tremor, bradykinesia, rigidity, and postural instability. With it's possible relation to degenerative dopamine-producing cells in the brain, administration of L-DOPA can increase the amount of synthesized dopamine in the nerve cell; direct treatment with dopamine is not sufficient as dopamine itself cannot pass the blood-brain barrier. However, only a small percentage of the dose actually reaches the nervous system, with the remaining majority being rapidly converted to dopamine in the blood stream. This dopamine-rich blood causes side effects of nausea, daytime sleepiness, orthostatic hypotension, involuntary movements, decreased appetite, insomnia, and cramping. Addition of a DDC inhibitor would block peripheral conversion to dopamine and allow a greater percentage of L-DOPA to reach the brain, causing an increase in brain dopamine levels, and diminishing the side effects of dopamine-rich blood.
Inhibitor Binding
The inhibitor carbiDOPA binds to the enzyme by forming a hydrazone linkage with the PLP cofactor through its hydrazine moiety [1].
The catechol ring buries itself deep within the active site cleft, with the 4' hydroxyl of the catechol ring hydrogen bonded to the hydroxyl group of Thr 82. Additionally, the highly conserved His 192 residue forms a hydrogen bond to the carboxylate group of carbiDOPA (with the carboxylate group of L-DOPA being even closer in binding range). Thus, His 192 is most likely a key residue in the enzyme's catalytic activity because a H192A mutation can cause complete loss of activity.