User:Brian Hernandez/DOPA Decarboxylase
From Proteopedia
(→'''Inhibitor Binding''') |
|||
| Line 24: | Line 24: | ||
==='''Inhibitor Binding'''=== | ==='''Inhibitor Binding'''=== | ||
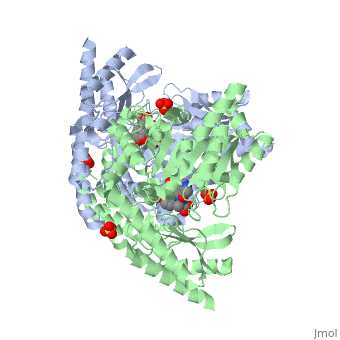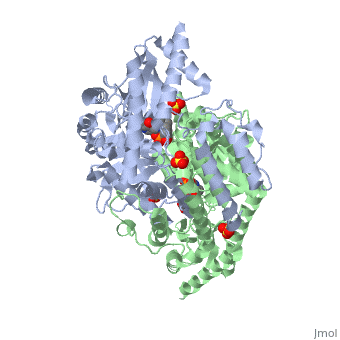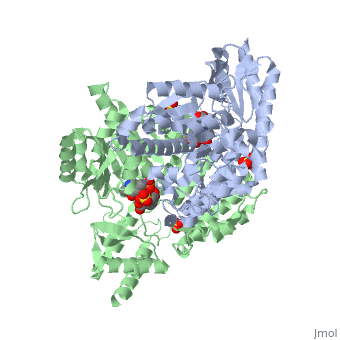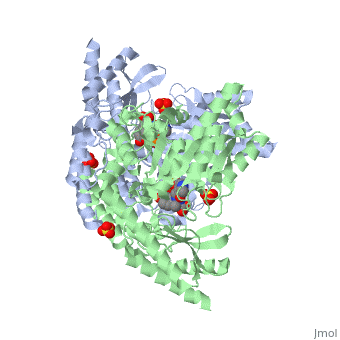
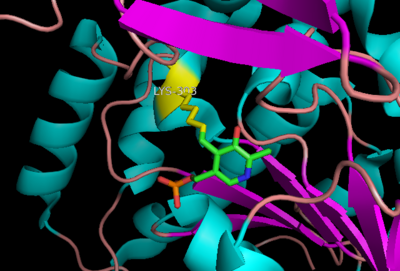
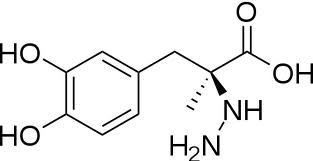
| - | The inhibitor '' | + | The inhibitor <scene name='DOPA_decarboxylase/Carbidopa/1'>carbiDOPA</scene> binds to the enzyme by forming a hydrazone linkage with the PLP cofactor through its hydrazine moiety <ref name=Burkhard>PMID: 11685243 </ref>. |
[[Image:carbidopa.jpeg|thumb|right|400px|'''carbiDOPA''']] The catechol ring buries itself deep within the active site cleft, with the 4' hydroxyl of the catechol ring hydrogen bonded to the hydroxyl group of Thr 82. Additionally, the highly conserved His 192 residue forms a hydrogen bond to the carboxylate group of carbiDOPA (with the carboxylate group of L-DOPA being even closer in binding range). Thus, His 192 is most likely a key residue in the enzyme's catalytic activity because a H192A mutation can cause complete loss of activity. | [[Image:carbidopa.jpeg|thumb|right|400px|'''carbiDOPA''']] The catechol ring buries itself deep within the active site cleft, with the 4' hydroxyl of the catechol ring hydrogen bonded to the hydroxyl group of Thr 82. Additionally, the highly conserved His 192 residue forms a hydrogen bond to the carboxylate group of carbiDOPA (with the carboxylate group of L-DOPA being even closer in binding range). Thus, His 192 is most likely a key residue in the enzyme's catalytic activity because a H192A mutation can cause complete loss of activity. | ||
Revision as of 06:01, 29 November 2011
| |||||||||||
DDC and Parkinson's Disease
Treatment
Parkinson's disease, a neurological disorder, can be characterized by tremor, bradykinesia, rigidity, and postural instability. With it's possible relation to degenerative dopamine-producing cells in the brain, administration of L-DOPA can increase the amount of synthesized dopamine in the nerve cell; direct treatment with dopamine is not sufficient as dopamine itself cannot pass the blood-brain barrier. However, only a small percentage of the dose actually reaches the nervous system, with the remaining majority being rapidly converted to dopamine in the blood stream. This dopamine-rich blood causes side effects of nausea, daytime sleepiness, orthostatic hypotension, involuntary movements, decreased appetite, insomnia, and cramping. Addition of a DDC inhibitor would block peripheral conversion to dopamine and allow a greater percentage of L-DOPA to reach the brain, causing an increase in brain dopamine levels, and diminishing the side effects of dopamine-rich blood.
Inhibitor Binding
The inhibitor binds to the enzyme by forming a hydrazone linkage with the PLP cofactor through its hydrazine moiety [1].
The catechol ring buries itself deep within the active site cleft, with the 4' hydroxyl of the catechol ring hydrogen bonded to the hydroxyl group of Thr 82. Additionally, the highly conserved His 192 residue forms a hydrogen bond to the carboxylate group of carbiDOPA (with the carboxylate group of L-DOPA being even closer in binding range). Thus, His 192 is most likely a key residue in the enzyme's catalytic activity because a H192A mutation can cause complete loss of activity.