ToxT
From Proteopedia
| Line 7: | Line 7: | ||
==Other Structural Features== | ==Other Structural Features== | ||
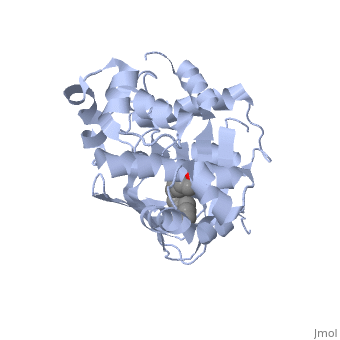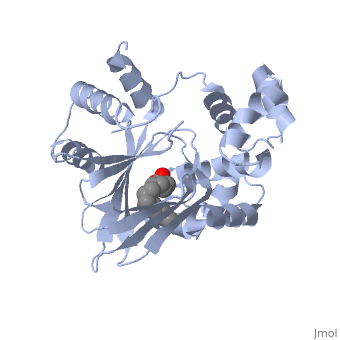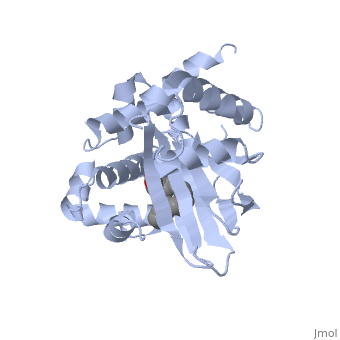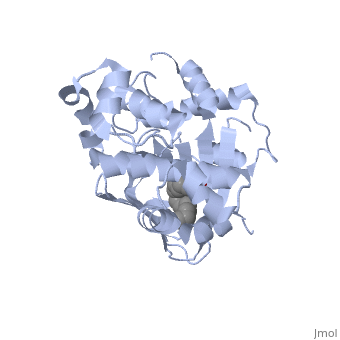
| - | <u>DNA-binding</u>. ToxT belongs to a family of transcriptional regulators headed by and known as AraC.<ref name="structure">PMID: 20133655</ref> The AraC family is characterized by a 100 amino acid region of sequence similarity that forms a <scene name='ToxT_Transcriptional_Regulator_in_Vibrio_cholerae/Two_hth_domains/1'>DNA-binding domain</scene> with two helix-turn-helix motifs ( | + | <u>DNA-binding</u>. ToxT belongs to a family of transcriptional regulators headed by and known as AraC.<ref name="structure">PMID: 20133655</ref> The AraC family is characterized by a 100 amino acid region of sequence similarity that forms a <scene name='ToxT_Transcriptional_Regulator_in_Vibrio_cholerae/Two_hth_domains/1'>DNA-binding domain</scene> with two helix-turn-helix motifs (one on either side of the black linker). <ref name="arac">PMID: 11282467</ref> The two HTH regions are linked by a very polar alpha helix (shown in black). The overall domain is located at the C-terminus.<ref name="structure">PMID: 20133655</ref> Assuming ToxT is similar in mechanism to other AraC proteins, helix six from HTH1 and helix nine from HTH2 become aligned with the help of helix seven (the polar linking region) to allow binding to major consecutive grooves of target DNA.<ref name="structure">PMID: 20133655</ref>. The conformation of helix seven is dependent on the ligand bound. |
<br/> | <br/> | ||
<br/> | <br/> | ||
Revision as of 22:17, 29 November 2011
The crystal structure of ToxT is resolved in monomeric form, after isolation from Vibrio cholerae strain O395.[1]
Introduction
ToxT is a molecule at the end of a transcriptional cascade that autoregulates the transcription of the primary virulence factors of Vibrio cholerae[3] and itself. ToxT is a cytoplasmic protein that is activated in turn by ToxR, which is itself activated by ToxS in response to environmental stimuli.[2] These two factors, cholera toxin (CT)[4] and the toxin co-regulated pilus (TCP), are instrumental in causing the disease cholera[5]. This is an intestinal infection resulting in massive water loss in the affected individual, causing extreme dehydration.[6]
| |||||||||||
Further Study
Conclusive results about what activates ToxT itself has not yet been found. The varying activity of ToxT dependent on the presence of cis-palmitoleate or other unsaturated fatty acids represents a detailed method of effective pathogenicity in humans, but may not be a reasonable target for drug treatment. By restricting transcription (and thus translation and protein production) of virulence genes until the bacterium is determined to be in a favorable location for infection, Vibrio cholerae avoids wasting energy producing virulence factors that will just be cleared by the intestine. This is a specific mechanism to ensure that the bacterium also injects CT and TCP where they will do the most damage, perpetuating the infection. [6]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Lowden MJ, Skorupski K, Pellegrini M, Chiorazzo MG, Taylor RK, Kull FJ. Structure of Vibrio cholerae ToxT reveals a mechanism for fatty acid regulation of virulence genes. Proc Natl Acad Sci U S A. 2010 Feb 16;107(7):2860-5. Epub 2010 Feb 1. PMID:20133655
- ↑ Kenneth Todar [1] Vibrio cholerae and Asiatic Cholera, Todar's Online Textbook of Bacteriology. Date of access: 2011-11-28.
- ↑ Martin RG, Rosner JL. The AraC transcriptional activators. Curr Opin Microbiol. 2001 Apr;4(2):132-7. PMID:11282467
- ↑ Weber GG, Klose KE. The complexity of ToxT-dependent transcription in Vibrio cholerae. Indian J Med Res. 2011 Feb;133(2):201-6. PMID:21415495
- ↑ Shakhnovich EA, Hung DT, Pierson E, Lee K, Mekalanos JJ. Virstatin inhibits dimerization of the transcriptional activator ToxT. Proc Natl Acad Sci U S A. 2007 Feb 13;104(7):2372-7. Epub 2007 Feb 5. PMID:17283330 doi:10.1073/pnas.0611643104
- ↑ Kenneth Todar [2] Vibrio cholerae and Asiatic Cholera, Todar's Online Textbook of Bacteriology. Date of access: 2011-11-28.
Proteopedia Page Contributors and Editors (what is this?)
Ingrid Youngworth, Yang Yang, Michal Harel, Alexander Berchansky, Jaime Prilusky