FK506 binding protein
From Proteopedia
| Line 22: | Line 22: | ||
{{Clear}} | {{Clear}} | ||
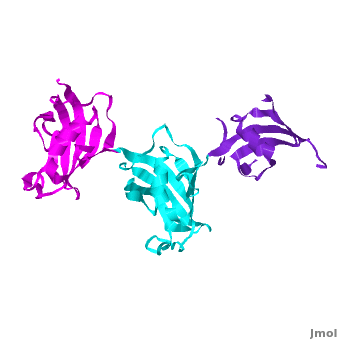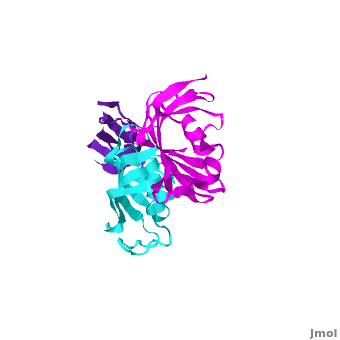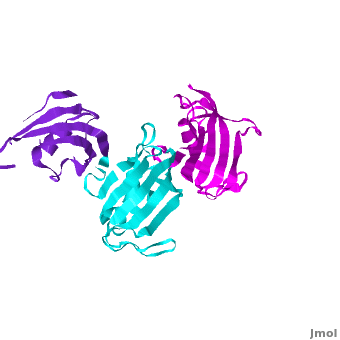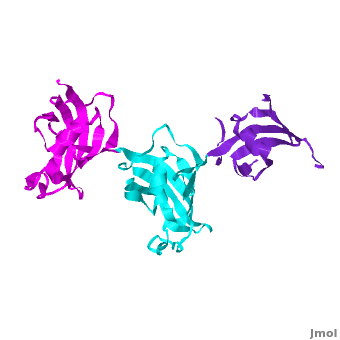
| - | The 3D structures of several FKBP family members from various species are solved, most of them comprise 1-2 FK domains (''e.g.'' human FKBP52), while wFKBP73 has 3 FK domains which is characteristic to plants. A sequence-based structure comparison between each of the 3 FK domains of wFKBP73 and the 2 FK domains of hFKBP52 ([[1q1c]]) was performed. All 3 FK domains of wFKBP73 adopt a typical FK fold exhibiting significant diversity when superimposed. They are arranged in a linear manner in space as observed in the 2 FK domains of hFKBP52. While the 2 FK domains of hFKBP52 are in the same orientation, the orientation between any 2 consecutive wFK73 domains is different than that between the two FK domains of hFKBP52. <scene name='3jym/Al/2'>Superposition</scene> of the <font color='blueviolet'><b>wFK73_1 (in blueviolet)</b></font> and <font color='cyan'><b>wFK73_2 (in cyan)</b></font> domains on hFK52_1 (in yellow) and <font color='blue'><b>hFK52_2 (in blue)</b></font> revealed that while <font color='cyan'><b>wFK73_2</b></font> is perfectly aligned with <font color='blue'><b>hFK52_2</b></font>, N-terminal <font color='blueviolet'><b>wFK73_1</b></font> does not align with hFK52_1 (yellow). Similarly, <scene name='3jym/Al/3'>superposition</scene> of the <font color='cyan'><b>wFK73_2</b></font> and <font color='magenta'><b>wFK73_3 (in magenta)</b></font> domains on hFKBP52 revealed that while <font color='cyan'><b>wFK73_2</b></font> is perfectly aligned with hFK52_1 (in yellow), <font color='magenta'><b>wFK73_3</b></font> does not align with <font color='blue'><b>hFK52_2</b></font>. This unique arrangement of wFKBP73 causes that the α-helices of <scene name='3jym/Al/4'>wFKBP73 3 FK domains</scene> are exposed on the same surface, while the <scene name='3jym/Al/5'>2 α-helices of hFK52</scene> are presented on opposite surfaces. | + | The 3D structures of several FKBP family members from various species are solved, most of them comprise 1-2 FK domains (''e.g.'' human FKBP52, known also as FKBP4), while wFKBP73 has 3 FK domains which is characteristic to plants. A sequence-based structure comparison between each of the 3 FK domains of wFKBP73 and the 2 FK domains of hFKBP52 ([[1q1c]]) was performed. All 3 FK domains of wFKBP73 adopt a typical FK fold exhibiting significant diversity when superimposed. They are arranged in a linear manner in space as observed in the 2 FK domains of hFKBP52. While the 2 FK domains of hFKBP52 are in the same orientation, the orientation between any 2 consecutive wFK73 domains is different than that between the two FK domains of hFKBP52. <scene name='3jym/Al/2'>Superposition</scene> of the <font color='blueviolet'><b>wFK73_1 (in blueviolet)</b></font> and <font color='cyan'><b>wFK73_2 (in cyan)</b></font> domains on hFK52_1 (in yellow) and <font color='blue'><b>hFK52_2 (in blue)</b></font> revealed that while <font color='cyan'><b>wFK73_2</b></font> is perfectly aligned with <font color='blue'><b>hFK52_2</b></font>, N-terminal <font color='blueviolet'><b>wFK73_1</b></font> does not align with hFK52_1 (yellow). Similarly, <scene name='3jym/Al/3'>superposition</scene> of the <font color='cyan'><b>wFK73_2</b></font> and <font color='magenta'><b>wFK73_3 (in magenta)</b></font> domains on hFKBP52 revealed that while <font color='cyan'><b>wFK73_2</b></font> is perfectly aligned with hFK52_1 (in yellow), <font color='magenta'><b>wFK73_3</b></font> does not align with <font color='blue'><b>hFK52_2</b></font>. This unique arrangement of wFKBP73 causes that the α-helices of <scene name='3jym/Al/4'>wFKBP73 3 FK domains</scene> are exposed on the same surface, while the <scene name='3jym/Al/5'>2 α-helices of hFK52</scene> are presented on opposite surfaces. |
{{Clear}} | {{Clear}} | ||
| Line 62: | Line 62: | ||
[[2gaq]], [[2pnu]]– hFKBP - NMR<br /> | [[2gaq]], [[2pnu]]– hFKBP - NMR<br /> | ||
[[1fkd]], [[1fkj]], [[2fke]], [[1qpf]], [[1qpl]] – hFKBP + immunosuppressant<br /> | [[1fkd]], [[1fkj]], [[2fke]], [[1qpf]], [[1qpl]] – hFKBP + immunosuppressant<br /> | ||
| + | [[2ppp]], [[2ppn]], [[2dg3]], [[1d6o]] – hFKBP<br /> | ||
[[1j4h]], [[1j4i]] – hFKBP + inhibitor<br /> | [[1j4h]], [[1j4i]] – hFKBP + inhibitor<br /> | ||
[[1b6c]] – hFKBP + TGF-B superfamily receptor I<br /> | [[1b6c]] – hFKBP + TGF-B superfamily receptor I<br /> | ||
Revision as of 10:44, 8 December 2011
FK506 binding protein (FKBP) is a prolyl isomerase related to the cyclophilins. FKBP is a folding chaperone for proteins containing prolines. FKBP12 binds the immunosuppressor tacrolimus (FK506) which is used against organ rejection.
Contents |
Wheat FKBP73
| |||||||||||
3D Structures of FKBP
FKPB3
3kz7 – hFKBP FK506-binding domain + immunosuppressant - human
FKPB4
1q1c – hFKBP
1n1a – hFKBP N terminal
1p5q - hFKBP C terminal
1qz2 – hFKBP + Hsp90 peptide
FKBP5
3o5d, 3o5e, 3o5f – hFKBP
3o5g, 3o5i, 3o5j, 3o5k - hFKBP FK506-binding domain
3o5l, 3o5m, 3o5o, 3o5p, 3p5q - hFKBP FK506-binding domain (mutant)
3o5r - hFKBP FK506-binding domain (mutant) + immunosuppressant
FKBP8
2f2d, 3ey6, 2awg - hFKBP FK506-binding domain
2d9f – hFKBP – NMR
2jwx - hFKBP N terminal - NMR
FKBP12
1eym – hFKBP (mutant)
1fkk – hFKBP
2gaq, 2pnu– hFKBP - NMR
1fkd, 1fkj, 2fke, 1qpf, 1qpl – hFKBP + immunosuppressant
2ppp, 2ppn, 2dg3, 1d6o – hFKBP
1j4h, 1j4i – hFKBP + inhibitor
1b6c – hFKBP + TGF-B superfamily receptor I
3fap – hFKBP + FKBP12-rapamycin associated protein
4fap - hFKBP + FKBP12-rapamycin associated protein + immunosuppressant
1tco - FKBP + Ser/Thr phosphatase B2 + immunosuppressant - bovine
1yat – FKBP + antagonist – yeast
FKBP26
3pr9, 3pra, 3prb, 3prd – FKBP – Methanocaldococcus jannaschii
FKBP59
1rot, 1rou – FKBP N terminal – NMR – rabbit
2kr7 – FKBP SlyD – NMR - Helicobacter pylori
2lgo – FKBP – NMR – Giardia lamblia
FKBP73
3jym - FKBP wheat