User:Dannielle Ryman/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | < | + | <applet load='1MP8' size='300' color='black' frame='true' align='right' caption='(FAK)Crystal structure of Focal Adhesion Kinase (FAK)''/> |
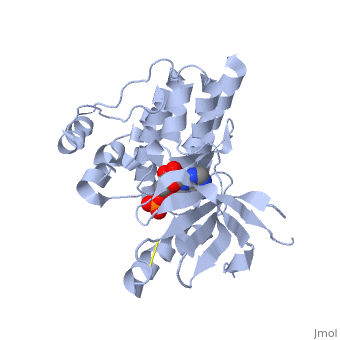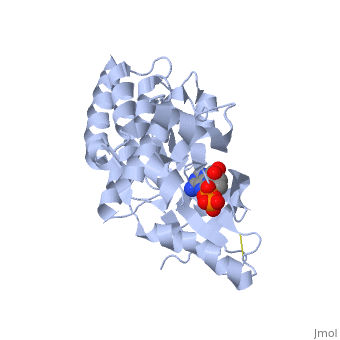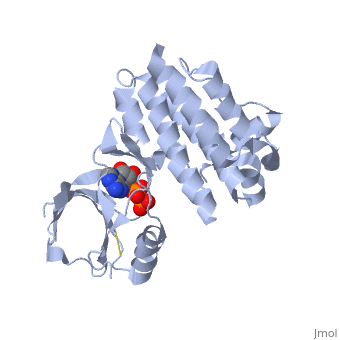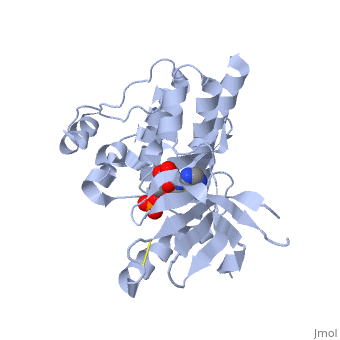
| + | <applet load='2IJM' size='300' color='black' frame='true' align='right' caption='Crystal Structure of Focal Adhesion Kinase Domain with 2 molecules in the Asymmetric Unit Complexed with ADP and ATP''/> | ||
| - | <Structure load='2ijm' size='300' frame='true' align='right' caption='Crystal Structure of Focal Adhesion Kinase Domain with 2 molecules in the Asymmetric Unit Complexed with ADP and ATP' scene='FAK Domain'/> | ||
===Background=== | ===Background=== | ||
Revision as of 23:01, 16 December 2011
|
|
Contents |
Background
Focal adhesion kinase (FAK) is a protein tyrosine kinase which is recruited at an early stage to focal adhesions and which mediates many of the downstream responses. FAK plays a very important role in integrin-mediated signaling and in modulating such processes as cell growth, differentiation, wound healing, and tumor metastasis.
Structure
Focal adhesion kinase (FAK) contains a FERM (protein 4.1, ezrin, radixin and moesin homology) domain, a kinase domain and a focal adhesion targeting (FAT) domain. The FERM domain mediates interactions of FAK with the epidermal growth factor (EGF) receptor, platelet-derived growth factor (PDGF) receptor, the ETK tyrosine kinase and ezrin, and the FERM domain can be conjugated to SUMO (small ubiquitin-related modifier) at Lys152. The FAT domain recruits FAK to focal contacts by associating with integrin-associated proteins such as talin and paxillin. It also links FAK to the activation of Rho GTPases by binding to guanine nucleotide-exchange factors (GEFs) such as p190 RhoGEF. FAK contains three proline-rich regions (PRR1–3), which bind Src-homology-3 (SH3) domain-containing proteins such as p130Cas, the GTPase regulator associated with FAK (GRAF) and the Arf-GTPase-activating protein ASAP1. FAK is phosphorylated (P) on several tyrosine residues, including Tyr397, 407, 576, 577, 861 and 925. Tyrosine phosphorylation on Tyr397 creates a Src-homology-2 (SH2) binding site for Src, phospholipase Cgamma (PLCgamma), suppressor of cytokine signalling (SOCS), growth-factor-receptor-bound protein 7 (GRB7), the Shc adaptor protein, p120 RasGAP and the p85 subunit of phosphatidylinositol 3-kinase (PI3K). Phosphorylation of Tyr576 and Tyr577 within the kinase domain is required for maximal FAK catalytic activity, whereas the binding of FAK-family interacting protein of 200 kDa (FIP200) to the kinase region inhibits FAK catalytic activity. FAK phosphorylation at Tyr925 creates a binding site for GRB2.Drug Target
References
1. Noble, Molecular Biophysics, 2001
2. Satyajit K. Mitra. Nature Reviews, 2005