Sandbox 212
From Proteopedia
(Difference between revisions)
| Line 22: | Line 22: | ||
<Structure load='1ndf' size='500' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' /> | <Structure load='1ndf' size='500' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' /> | ||
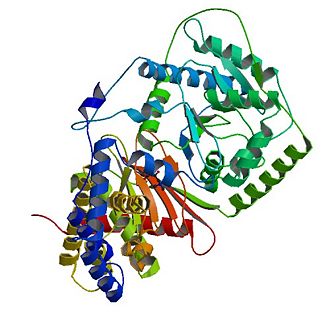
| - | The tertiary structure of CAT consists of 20 α-helices (α1-α20) and 16 β-strands (named β1-β16) which are arranged into two equally sized domains (N and C domains ). | + | The tertiary structure of CAT consists of 20 α-helices (α1-α20) and 16 β-strands (named β1-β16) which are arranged into two equally sized domains (N and C domains ). |
== Substrate binding and mechanism == | == Substrate binding and mechanism == | ||
Revision as of 11:30, 23 December 2011
Carnitine acyltransferases are a large family of enzymes that play a main role in cellular energy metabolism, i.e. fatty acid oxidation. These enzymes catalyze the reversible exchange of acyl groups between Coenzyme A and carnitine. Carnitine acyltransferases include three different classes of enzymes which are known as carnitine acetyltransferases (CrATs), carnitine octanoyltransferases (CrOTs) and carnitine palmityltransferase (CPTs). The three classes of differ in their acyl group specificity as well as their localization.[1]