Sandbox 212
From Proteopedia
| Line 28: | Line 28: | ||
== Overall structure of Carnitine acetyltransferase == | == Overall structure of Carnitine acetyltransferase == | ||
| - | <Structure load='1ndf' size='500' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' /> | ||
| - | There are two distinct classes of HMGRs, class I HMGRs, which are only found in eukaryotes and are membrane bound and class II HMGRs, which are found in prokaryotes and are soluble.<ref>PMID:11349148</ref> HMGR contains 8 transmembrane domains that have yet to be successfully crystallized, which anchor the protein to the membrane of the endoplasmic reticulum.<ref name="Roitelman"/> The catalytic portion of human HMGR forms a tetramer, with the individual monomers winding around each other.<ref name="Roitelman">PMID:1374417</ref> Within the tetramer, the monomers are arranged into <scene name='HMG-CoA_Reductase/1dq8_2_dimers/3'>two dimers</scene>, each of which contains <scene name='HMG-CoA_Reductase/1dq8_2_active_sites/2'>two active sites </scene>which are formed by residues form both monomers. Each monomer contains <scene name='HMG-CoA_Reductase/1dq8_star3_domains/2'>three domains </scene>, the <scene name='HMG-CoA_Reductase/1dq8_n_domain/2'>N-domain</scene>, the <scene name='HMG-CoA_Reductase/1dq8_l_domain/1'>L-Domain</scene>, and the <scene name='HMG-CoA_Reductase/1dq8_s_domain/1'>S-Domain</scene>. The L-domain is unique to HMGRs while the S-domain, which forms the binding site for NADP, resembles that of [[ferredoxin]]. The S and L domains are connected by a <scene name='HMG-CoA_Reductase/1dq8_cis_loop/5'>“cis-loop”</scene> which is essential for the HMG-binding site.<ref name="Roitelman"/> Salt bridges between residues R641 and E782 as well as <scene name='HMG-CoA_Reductase/1dq8_cis_loop/4'>hydrogen bonds</scene> between E700 and E700 on neighboring monomers compliment the largely hydrophobic dimer-dimer interface.<ref name="Roitelman"/> | ||
| - | <br /> | ||
| - | |||
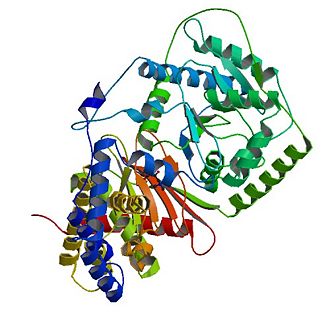
The tertiary structure of CAT consists of 20 α-helices (α1-α20) and 16 β-strands (named β1-β16) which are arranged into two equally sized domains (N and C domains ). | The tertiary structure of CAT consists of 20 α-helices (α1-α20) and 16 β-strands (named β1-β16) which are arranged into two equally sized domains (N and C domains ). | ||
== Substrate binding and mechanism == | == Substrate binding and mechanism == | ||
| + | |||
Revision as of 21:26, 23 December 2011
|
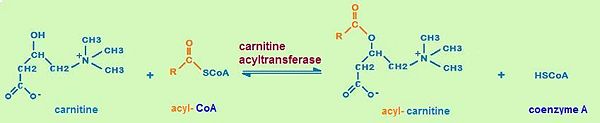
Carnitine acyltransferases are a large family of enzymes that play a main role in cellular energy metabolism, i.e. fatty acid oxidation. These enzymes catalyze the reversible exchange of acyl groups between coenzyme A and carnitine. Carnitine acyltransferases include three different classes of enzymes which are known as carnitine acetyltransferases (CrATs), carnitine octanoyltransferases (CrOTs) and carnitine palmityltransferase (CPTs). The three classes differ in their acyl group specificity as well as their localization. [1] In this entry we will focus on the structure of carnitine acetyltransferase as a representitive of carnitine acyltransferases. Determining the structure and thus the molecular basis for fatty acid transfer is needed for drug development.Being major enzymes in fatty acid oxidation carnitine acyltransferases are viewed as promising targets which can be used to develop successful therapeutics against type 2 diabetes, obesity and other human diseases.
Contents |
Biological function
- Differences within carnitine acyltransferase family members
Carnitine acyltransferases carry out pivotal biological functions. All members of carnitine acyltransferases catalyze the same reversible reaction:the exchange of acyl groups between carnitine and coenzyme A (CoA). This is in accord with the fact, that the catalytic domains of all the carnitine acyltransferases are well conserved. However the three known classes of carnitine acyltransferases differ in their acyl group specificity, subcellular localization, tissue distribution, and physiological function(3). Carnitine acetyltransferase (CrATs) prefere short chain fatty acids as substrates and are localized in the mitochondrial matrix, the endoplasmic reticulum, and the peroxisome. Carnitine octanoyltransferases (COTs) show medium chain fatty acid substrate preference and are mainly found in peroxisomes. Carnitine palmitoyltransferase (CPTs) have long chain fatty acids substrate preference and are found in the outer mitochondrial membrane and the mitochondrial matrix.
- The role of carnitine acyltransferases in fatty acid oxidation
The most important biological function of carnitine acyltransferases is the transport of fatty acids for β-oxidation(2).Fatty acids are oxidized for energy production in the mitochondrial matrix by a process called β- oxidation. The major site of fatty acid accumulation, however, is the cytoplasm of the cells. Hence, in order to provide energy, fatty acids have to be transported from the cytoplasm across the inner mitochondrial membrane into the mitochondrial matrix. The carnitine shuttle , a transport chain that consists of three enzymatic reactions, helps fatty acids to pass the mitochondrial membrane. Carnitine acyltransferases (CrATs, CrOTs, CPTs) are part of the carnitine shuttle.
The first step of the carnitine shuttle is the activation of fatty acids. They are transformed into the activated form (= acyl CoA) by the formation of a thioester linkage between the fatty acid carboxyl group and the thiol group of coenzyme A. The second step of the carnitine shuttle is catalyzed by carnitine acyltransferases. These enzymes (especially CPT) facilitate the transport of fatty acids by conjugating them to carnitine. In this reaction an acyl group is transferred from the sulfur atom of CoA to the hydroxyl group of carnitine. The product is acyl- carnitine. The third step of the carnitine shuttle is also catalyzed by carnitine acyltransferases (especially CPT) in the mitochondrial matrix. In this reaction the acyl group of acyl- carnitine ester is transferred back to CoA to form acyl- CoA.
Overall structure of Carnitine acetyltransferase
The tertiary structure of CAT consists of 20 α-helices (α1-α20) and 16 β-strands (named β1-β16) which are arranged into two equally sized domains (N and C domains ).