Sandbox 215
From Proteopedia
(→Proteopedia Page Contributors and Editors) |
(→References) |
||
| Line 73: | Line 73: | ||
==References== | ==References== | ||
| - | * Qiu X, Mistry A, Ammirati MJ, Chrunyk BA, Clark RW, Cong Y, Culp JS, Danley DE, Freeman TB, Geoghegan KF, Griffor MC, Hawrylik SJ, Hayward CM, Hensley P, Hoth LR, Karam GA, Lira ME, Lloyd DB, McGrath KM, Stutzman-Engwall KJ, Subashi AK, Subashi TA, Thompson JF, Wang IK, Zhao H, Seddon AP. Crystal Structure of cholesteryl ester transfer protein reveals a long tunnel and four bound lipid molecules. Nature Structural & Molecular Biology. 2007 Feb;14(2):106-13. Epub 2007 Jan 21. [http://www.ncbi.nlm.nih.gov/pubmed?term=17237796 PMID: 17237796] [http://www.nature.com.scd-rproxy.u-strasbg.fr/nsmb/journal/v14/n2/full/nsmb1197.html doi:10.1038/nsmb1197] | + | * Qiu X, Mistry A, Ammirati MJ, Chrunyk BA, Clark RW, Cong Y, Culp JS, Danley DE, Freeman TB, Geoghegan KF, Griffor MC, Hawrylik SJ, Hayward CM, Hensley P, Hoth LR, Karam GA, Lira ME, Lloyd DB, McGrath KM, Stutzman-Engwall KJ, Subashi AK, Subashi TA, Thompson JF, Wang IK, Zhao H, Seddon AP. Crystal Structure of cholesteryl ester transfer protein reveals a long tunnel and four bound lipid molecules. Nature Structural & Molecular Biology. 2007 Feb;14(2):106-13. Epub 2007 Jan 21. [http://www.ncbi.nlm.nih.gov/pubmed?term=17237796. PMID: 17237796] [http://www.nature.com.scd-rproxy.u-strasbg.fr/nsmb/journal/v14/n2/full/nsmb1197.html doi:10.1038/nsmb1197] |
| - | * James A Hamilton & Richard J Deckelbaum | + | * James A Hamilton & Richard J Deckelbaum. Crystal structure of CETP: new hopes for raising HDL to decrease risk of cardiovascular disease? Nature Structural & Molecular Biology 14, 95 - 97 (2007). [https://www-ncbi-nlm-nih-gov.scd-rproxy.u-strasbg.fr/pubmed/17277799 PMID: 17277799] [http://www.nature.com.scd-rproxy.u-strasbg.fr/nsmb/journal/v14/n2/full/nsmb0207-95.html doi:10.1038/nsmb0207-95] |
| + | |||
| + | * Cao G, Beyer TP, Zhang Y, Schmidt RJ, Chen YQ, Cockerham SL, Zimmerman KM, Karathanasis SK, Cannady EA, Fields T, Mantlo NB. Evacetrapib is a novel, potent, and selective inhibitor of cholesteryl ester transfer protein that elevates HDL cholesterol without inducing aldosterone or increasing blood pressure. The Journal of Lipid Research, December 2011. [https://www-ncbi-nlm-nih-gov.scd-rproxy.u-strasbg.fr/pubmed/21957197 PMID: 21957197]. [http://www.jlr.org.scd-rproxy.u-strasbg.fr/content/52/12/2169.long doi: 10.1194/jlr.M018069] | ||
== Proteopedia Page Contributors and Editors == | == Proteopedia Page Contributors and Editors == | ||
BLEU Mélusine, GOEPFERT Laetitia | BLEU Mélusine, GOEPFERT Laetitia | ||
Revision as of 15:45, 28 December 2011
| |||||||||
| 2obd, resolution 2.10Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , , , , , , , | ||||||||
| Gene: | CETP (Homo sapiens) | ||||||||
| |||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
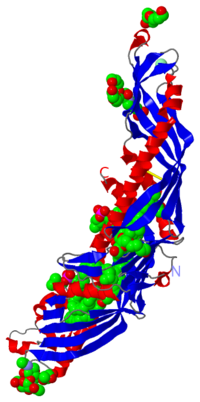
Cholesteryl Ester Transfer Protein is a hydrophobic plasma glycoprotein which is implicated in the transport of cholesteryl esters from the atheroprotective high-density lipoproteins (HDL) to the atherogenic lower-density lipoproteins (LDL). CETP also mediates the transport of triglycerides from LDL to HDL. The cristal structure of CETP at 2,2Å resolution shows a long tunnel traversing the core of the molecule and has two distinct large openings allowing lipids access.
Contents |
Structure
Overview of the structure
|
CETP is a 476 amino acid residues protein which has an elongated “boomerang shape” with dimensions of 135Å X 30Å X 35Å. She has a molecular mass of 74 kDa. CETP is a highly hydrophobic and glycosylated protein: in fact 28% of her mass is attributed to N-glycosylation at specific residues : 88, 240, 341 and 396.
CETP's structure can be divided into four structural units:
- At each end of the protein there is a barrel, which is constitued of highly twisted ß-sheet and two helices called A and B at the and A', B' at C-terminal extremity. Helices B and B' are longer than helices A and A'.
- Between the two barrels there is a central which is constitued of six antiparallel strands.
- At the extremity there is a distorted amphiphathic helix called which is an extension of C-teminal interacting with N-terminal residues.
Four lipid binding sites
CETP's structure reveals a 60 Å long hydrophobic tunnel which traverses the core of the molecule and contains four lipid binding sites: two neutral lipids binding sites in the middle of the tunnel and two phospholipids binding sites (one at each end). The center of the tunnel which is called the “neck” is 10 Å wide and 5 Å high that is large enough to permit the passage of neutral lipid.
A wall of β-sheets underneath the lipids and a layer of helices above the lipids forms the longest tunnel that it exists in lipid-binding and lipid-transfer protein.
Cholesteryl ester 1 (CE1) is situated between the N barrel and the central β-sheet. This binding site is mostly composed of hydrophobic residues and only a few polar. CE1 is too far away from the Ser 230 to establish a hydrogen bond but CE1 can establish some π-starking interaction.
Cholesteryl ester 2 penetrates deeper into the barrel than CE1 and resides between the central β-sheet and the C-barrel. This site contains even fewer polar groups than CE1 binding site. That's why CE2 is not able to make any hydrogen-bonding or π-starking interaction.
The N-opening of the tunnel is 10 Å wide and 5 Å high whereas the C-opening is 13 Å X 5 Å. The C-opening is a little bit larger but both are large enough to allow lipid access. Each opening of the tunnel is plugged by one phospholipid which buries its hydrophobic acyl chain inside the tunnel and its hydrophilic head groups to the solvent.
Helix X and Ω flaps
Some mobile structures located near tunnel openings can facilitate the lipid transfer. The belongs to the C-terminal domain and thanks to its Gly462-Phe463-Pro464 groupment is flexible. It is an amphiphathic helix. The hydrophobic face of helix X interacts with phosphatidylcholine 1 located at the N-terminal in order to form an apolar path allowing the access of neutral lipids to the tunnel. Mutations on the hydrophobic face of helix X reduce transfer activities whereas mutations on the polar side do not have any effects on transfer activities. These results prove that helix X plays an important role in transferring neutral lipid from lipoprotein to CETP. Near the C-opening, there are also two Ω flaps: Ω1 and Ω2. These flaps are linked through the starking interaction between the Phe292 and Ph350. The flap Ω1 interacts with the oleoyl tail of the cholesteryl ester 2 in order to protect the lipid from aqueous solvent exposure. Ω1 flap also helps the exchange of lipids through the C opening.
Mechanism allowing neutral-lipid and phospholipid transfer
In the plasma circulation, CETP often binds HDL and engages the tranfer of neutral lipids, such as cholesteryl ester and triglyceride among lipoprotein particles. The concave structure of CETP which is the site of the N and C openings, helix X and the Ω1 flap is the only surface able to bind a lipoprotein, other surfaces of CETP are not able to bind them. It indicates that CETP can bind only one lipoprotein at a time. It means that CETP operates as carrier: CETP accepts neutral lipids from a donor particule, then travels them through the acqueous phase and releases them to an acceptor particule.
Binding to a HDL particle, which is cholesteryl ester rich, allows CETP to fill with cholesteryl esters, because one or two cholesteryl esters can enter the tunnel and an equal amount of triglyceride is deposited into HDL. Then the tunnel is refilled with two phospholipid (one at each end) that permits the protein to dissociate from HDL and to retunr to the acqueous phase. CETP also adopts a structural change by twisting its barrel around the central β-sheet in order to bind VLDL particules which are larger than HDL particules. Binding to a VLDL particle, which is triglyceride rich, permits the release of the bound phospholipid. That allows one or two triglycerides to enter the tunnel and an equal amount of cholesteryl ester can be deposit into VLDL. The triglyceride-bound dissociates from VLDL. It carries two phospholipids from the surface of VLDL and travels through the acqueous plasma in order to rebind a HDL particle. Binding to a HDL particle, permits the release of the bound phospholipid and the cycle can continue.
CETP inhibitors
Cholesterol is the major lipid component of the plasma membrane of the animal cells. Cholesterol is also a constituent of lipoprotein complexes in the blood and one of the constituents of lipoprotein complexes of the plaques that form on the blood vessels by atherosclerosis.
HDL particles are considered as “good cholesterol” because they are able to remove cholesterol from peripheral tissues back to the liver via the plasma, in which they are degraded. This action prevents from the accumulation of cholesterol in the plasma. Unlike to HDL, LDL particles are often called “bad cholesterol”. They enable the transport of cholesterol from its place of secretion into the cells of the body (within the bloodstream). A high rate of LDLs leads to the deposition of cholesterol as plaque on artery walls. This can result in cardiovascular problems. Therefore, the inhibition of CETP could hinder the transport between HDL and LDL and so increases the HDL-C and decreases the VLDL-C. This action is a possible solution of atherosclerosis.
Natural inhibitors
In the human plasma some natural inhibitors of CETP can be found: like Apo-F which suppresses the transfer involving LDL and Apo-CI, which his main role is to inhibit the CETP probably by altering the elecric charge of HDL.
Pharmaceutical inhibitors
Pharmaceutical industrie tries to develop an inhibitor of CETP in order to decrease the risk of cardivascular diseases. The goal of these inhibitors is to increase the concentration of HDL and decrease the concentration of LDL by blocking CETP. Several inhibitors were found. The first was Torcetrapib followed by Anacetrapib, Dalcetrapib and the last one Evacetrapib. Torcetrapib succeeds in increasing the level of HDL, but his action develops side effects such as an increase of the blood pressure and the concentration of Sodium, Bicarbonate and aldosterone. That causes the death of many persons at the stage-III of the clinical trial. That's why this inhibitor was abort. Unlike to torcetrapib, the other do not present any side effect for the moment, but still are in clinical trial. Evacetrapib seems to give the more promising results.
External ressources
References
- Qiu X, Mistry A, Ammirati MJ, Chrunyk BA, Clark RW, Cong Y, Culp JS, Danley DE, Freeman TB, Geoghegan KF, Griffor MC, Hawrylik SJ, Hayward CM, Hensley P, Hoth LR, Karam GA, Lira ME, Lloyd DB, McGrath KM, Stutzman-Engwall KJ, Subashi AK, Subashi TA, Thompson JF, Wang IK, Zhao H, Seddon AP. Crystal Structure of cholesteryl ester transfer protein reveals a long tunnel and four bound lipid molecules. Nature Structural & Molecular Biology. 2007 Feb;14(2):106-13. Epub 2007 Jan 21. PMID: 17237796 doi:10.1038/nsmb1197
- James A Hamilton & Richard J Deckelbaum. Crystal structure of CETP: new hopes for raising HDL to decrease risk of cardiovascular disease? Nature Structural & Molecular Biology 14, 95 - 97 (2007). PMID: 17277799 doi:10.1038/nsmb0207-95
- Cao G, Beyer TP, Zhang Y, Schmidt RJ, Chen YQ, Cockerham SL, Zimmerman KM, Karathanasis SK, Cannady EA, Fields T, Mantlo NB. Evacetrapib is a novel, potent, and selective inhibitor of cholesteryl ester transfer protein that elevates HDL cholesterol without inducing aldosterone or increasing blood pressure. The Journal of Lipid Research, December 2011. PMID: 21957197. doi: 10.1194/jlr.M018069
Proteopedia Page Contributors and Editors
BLEU Mélusine, GOEPFERT Laetitia