User:Gourinchas Geoffrey/Sandbox 205
From Proteopedia
| Line 37: | Line 37: | ||
[http://en.wikipedia.org/wiki/Glycosylation The glycosylation] of this hormone allows his solubilisation in the blood and his carry towards his target, the bone marrow. | [http://en.wikipedia.org/wiki/Glycosylation The glycosylation] of this hormone allows his solubilisation in the blood and his carry towards his target, the bone marrow. | ||
| + | |||
| + | |||
| + | |||
| + | |||
Current revision
|
The Erythropoietin hormone is a glycoprotein which is involved in Erythropoiesis, which is the red blood cells production [1]. It allows the differenciation of the erythrocyte precursors in the bone marrow. Thus, Erythropoietin has a fundamental biological role in the regulation of the production of red blood cells and, thus, at the level of the oxygenation of the blood. Erythropoietin is thus a substance produced naturally by the body and primarily used for its therapeutic properties. Indeed, thanks to its properties, this hormone gives new life to thousands of patients suffering of anemia.
Contents |
Biological role
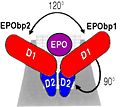
The gene of the erythropoietin was cloned at the man's, the mouse and the monkey. It is located at the man's on the chromosome 7 in the region q21. It contains 5 exons and 4 introns. The gene is very preserved according to the species. It exists 94 % of homology between the human gene and the monkey gene and 80 % of homology enters the murin gene and the human gene. Erythropoietin is a glycosylated hormone mainly secreted in the kidneys (90%) at the level of the cortex and of external medullary, and little in the liver. This hormone acts on the target cells by the intermediary of a specific receptor essentially on the erythroblastic progenitor, and a little on megacaryocyte progenitor. Through experiences of connection with the market hormone, it was demonstrated the existence of a single receptor (high affinity) or two types of receptor of high and low affinity. After the binding with its receptor, the Erythropoietin specifically leads the phosphorylation of the receptor on the tyrosine, from the first minutes which follow its connection.
The secretion of this hormone depend of the partial pressure of dioxygen in the kidney cells. The rate of Erythropoietin increases when there is a tissular hypoxia which origin can be anemic, secondary in an obstructive lung syndrome, in a left-right cardiac shunt or, much more rarely, in an abnormality of the affinity of the haemoglobin for oxygen. Thus needs of dioxygen of the tissues increase and thus the secretion of Erythropoietin is stimulated.
On the contrary, an increase of the pressure of dioxygen decrease the secretion of Erythropoietin. To increase the number of red blood cells, this hormone stimulate the proliferation of stem cells precursor of erythrocyte in the bone marrow which will became red blood cells.
Regulation of the Erythropoietin synthesis
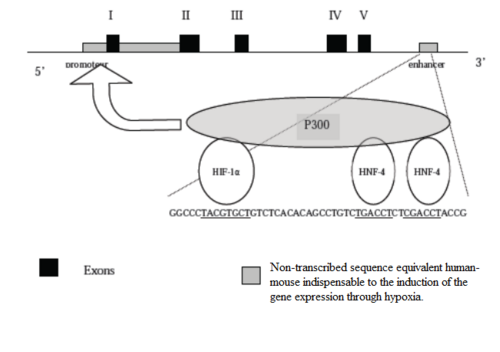
It was demonstrated hypoxia induced an important synthesis of mRNA of Erythropoietin. Thus the regulation of this synthesis occurs at tanscriptional level. There is no storage place for this mRNA or protein in the body also hypoxia acts directly on the level of the genes expression. We can observed at the end of 4 at 8 hours a peak of mRNA synthesis coding for Erythropoietin with an amplitude proportional to the degree of hypoxia. In comparing the non-coding sequences of the human erytrhopoietin gene with the murine erythropoietin gene, it was identified three segments very preserved: the promotor located in 5', the first intron and a region of 120 bp located downstream to the polyadenylation site (enhancer). It was demonstrated that during a hypoxia, only the promotor and the enhancer sequence are indispensable to the induction of the gene expression. There are three proteins which are involved in the promotor activation: HIF-1α, HNF-4 and p300. These proteins join and settle in a precise sequence of bases situated in the portion enhancer. The binding of this complex leads conformational modifications allowing the interaction of p300 with the sequence promotor.
It exist regulator elements upstream the complex HIF-1α/HNF-4/p300. HIF-1α is a cytosolic protein. Its passage in the nucleus allows the formation of the complex HIF-1α/HNF-4/p300 and thus the activation of erythropoietin gene transcription. The oxidized shape of HIF-1α is quickly recognized and degraded by a enzymatic complex called proteasome. In the cytosol threre is a complex protein-heme which used ferrous and ferric ions in their reaction of oxydo-reduction. When it interract with the dioxygene, the heme allows the liberation of superoxides ions which have a powerful oxydative power. These ions oxidize HIF-1α and thus provokes its degradation. In the presence of dioxygen, the gene promotor of the erythropoietin is not thus activated. In the absence of dioxygen, HIF-1α is not oxidized, crosses in the nucleus and allows the formation of the complex HIF-1α/HNF-4/p300.The glycosylation of this hormone allows his solubilisation in the blood and his carry towards his target, the bone marrow.
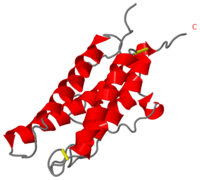
Structure
| |||||||||||
Application in medicine
Unfortunately, the production of Erythropoietin in the laboratory has long been impossible, because the concentration of the hormone is extremely low in the urine or human blood, thus a natural production was impossible. Today, the development of human recombinant synthesis Erythropoietin allows the treatment of anemic diseases. It is in particular possible to look after the resultant anemia of the chronic renal insufficiency. However, the treatment through intravenous way can lead to the arterial high blood pressure. It is for it that was developed the recombinant Erythropoietin injection by subcutaneous way which allows to avoid the principal secondary effects. So, recombinant Erythropoietin have received the approval for the treatment of renal insufficiency anemia at the hemodialysis patient and in predialysis by the intravenous way and the subcutaneous way.
It also exists insufficiency of Erythropoietin production in certain cases of anemia with preserved renal function. It is the case in the inflammatory anemia of the rheumatoid polyarthritis where an improvement of the anemia in been able to be obtained by treatment with recombinant erythropoietin.
Essays led to patients affected by cancer, where the mechanism of the anemia is largely inflammatory, showed that between 30 and 50 % of the patients answer the treatment. No effect of the treatment was noticed on the tumoral growth as well as on the cellular proliferation.
At the patients affected by the AIDS, the treatment with Zidovudine can aggravated the anemia. It was observed that a treatment with the recombinant erythropoietin reduced the transfusional needs for the patients.
For the premature newborn children presenting a severe anemia resulting from an incapacity of production of erythropoietin, a treatment with the recombinant erythropoietin shows a reduction of the transfusional needs and to pull a stabilization of the rate of haemoglobin.
However, the results obtained in these various anemia are insufficiently convincing to justify an extension of the indication of the treatment by the recombinant Erythropoietin.
See also
References
- ↑ Wolfgang Jelkmann and Eric Metzen, Erythropoietin in the control of red cell production, ANNALS Of ANATOMY
- ↑ Douglas D. Banks, The Effect of Glycosylation on the Folding Kinetics of Erythropoietin, J. Mol. Biol. (2011) 412, 536–550
- ↑ Por-Hsiung Lai, RicharEd verett, Fung-Fang Wang, Tsutomu Arakawa, and Eugene Goldwas, Structural Characterization of Human Erythropoietin, THE JOURNAL OF BIOLOGICAL CHEMISTRY (1986) vol.261 N°7 3116-3121
- ↑ Boissel JP, Lee WR, Presnell SR, Cohen FE, Bunn HF. Erythropoietin structure-function relationships. Mutant proteins that test a model of tertiary structure. J Biol Chem. 1993 Jul 25;268(21):15983-93. PMID:8340419
- ↑ Syed, Rashid S., Scott W. Reid, Cuiwei Li, Janet C. Cheetham, Kenneth H. Aoki, Beishan Liu, Hangjun Zhan, Timothy D. Osslund, Arthur J. Chirino, Jiandong Zhang, Janet Finer-Moore, Steven Elliott, Karen Sitney, Bradley A. Katz, David J. Matthews, John J. Wendoloski, Joan Egrie, and Robert M. Stroud. 1998. Efficiency of Signalling through Cytokine Receptors Depends Critically on Receptor Orientation. Nature 395: 511-516.
- ↑ Wilson IA, Jolliffe LK. The structure, organization, activation and plasticity of the erythropoietin receptor. Curr Opin Struct Biol. 1999 Dec;9(6):696-704. PMID:10607675
- ↑ Yoshimura A, Arai K. Physician Education: The Erythropoietin Receptor and Signal Transduction. Oncologist. 1996;1(5):337-339. PMID:10388012
Proteopedia Page Contributors and Editors.
Gourinchas Geoffrey and Williame Maïté