Sandbox 208
From Proteopedia
(→External Resources) |
|||
| Line 70: | Line 70: | ||
The major effect of both mutations could lead to a decrease of the Rab pool available for synaptic vesicles cycling and neurotransmitter release. | The major effect of both mutations could lead to a decrease of the Rab pool available for synaptic vesicles cycling and neurotransmitter release. | ||
| - | = External | + | = External resources = |
*[http://www.rcsb.org/pdb/explore.do?structureId=3CPH Crystal structure of Sec4 in complex with Rab-GDI] | *[http://www.rcsb.org/pdb/explore.do?structureId=3CPH Crystal structure of Sec4 in complex with Rab-GDI] | ||
Current revision
| |||||||||
| 3cpi, resolution 2.30Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Related: | 3cph, 3cpj | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Rab-Guanosine biphosphate Dissociation Inhibitor (RabGDI) belongs to the transport cytoplasmic protein group. RabGDI was firstly isolated from bovine brain and three isoforms were isolated (α, β, γ). However, in the yeast Saccharomyces cerevisiae, only one isoform was identified: Gdi1p.
In cells, vesicular traffic through the exocytic and endocytic pathways involves the activity of small Rab superfamilly GTPases which are named Rab proteins. Rab proteins exist in two different conformations: an active GTP bound conformation and an inactive GDP bound conformation. The process of switching between these two conformations requires a multitude of interacting and also regulatory proteins. Rab active form interacts with its effectors i.e GTPases activating proteins. On the contrary, the inactive conformation is recognized by guanine nucleotide exchange factors (GEF) and regulators proteins including GDI. GDI is an inhibitory protein which extracts prenylated Rab proteins from membranes at the end of their cycle of activity and facilitates their delivery to the donor membranes. GDI is indispensable for the vesicular transport machinery functioning: its deletion is lethal. In this entry, we only focus on the structure of the complex between GDI and a doubly prenylated Rab proteins.[1]
Contents |
Biological function[2] [3] [4]
- An essential step: Rab prenylation
All Rabs undergo posttranslational modifications by prenylation. Prenylation is essential for Rab function and membrane association but also for its binding to GDI. In most cases, this post-translationally modification consists in the attachment of geranylgeranyl lipid groups on two C-terminus cysteines. Geranylgeranylation is catalyzed by the prenyltransferase, geranylgeranyltransferase type II (GGTrII), a heterodimer containing α- and β-subunits. GGTrII requires the activity of an accessory protein, Rab escort protein (REP). REP is able to bind newly synthesized Rabs and to mediate their delivery to GGTrII.
- GDI versus REP
GDI does not facilitate Rabs prenylation since it lacks the geranylgeranyl transferase binding site. It is involved in Rabs recycling. Its function consists in retrieving Rab in the GDP bound form from the membrane and delivers it to the cytosol. Thus, GDI controls the Rabs distribution between cytosol and membranes. GDI is stably associated only with GDP loaded and prenylated Rab proteins. Both REP and GDI are considered as Rab molecular chaperones. They have similar organization, in particular, their Rab binding interface which is well conserved.
Structure[5]
| |||||||||||
Catalytic mechanism[6]
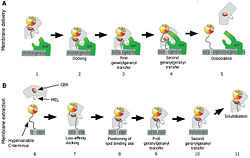
Initially, Rab is anchored to a target membrane via its lipid moieties. After interactions with its effectors, it returns to the GDP bound form. There is a primary recognition between Rab and GDI: the GTPase domain of Rab is recognized by GDI. GDI binds to Rab via the RBP, forming a low affinity complex. Then, the Rab conserved C terminal AXA box is recognized and bound by the GDI CCR, increasing the complex affinity. Interactions between CCR and the hydrophobic residues of the AXA box lead to a conformation change of the domain II. The GDI lipid binding pocket opens. It is located in the vicinity of the Rab lipid anchor, leading to a favorable situation for lipid transfer. Firstly, the first extracted lipid initially binds to the superficial lipid binding site leading to the formation of a transient high affinity complex still anchored in the membrane. Secondly, this complex is converted into a soluble complex by coordinating transfer of the GDI-bound lipid into the buried binding site. This event facilitates flipping of the second lipid from the membrane to the surface binding site. Thus, a high affinity Rab-GDI complex is formed and released from the membrane. GDI transports and then mediates the delivery of prenylated Rab to another target membrane. GDI leads to the docking of Rab via a protein interaction with the protein GDI. The docked complex undergoes a conformational change. This leads to the transfer of the first and then the second geranylgeranyl moiety into the membrane and subsequently to the release of the Rab C-terminus from the CBR. Finally, the Rab protein enters its functional cycle whereas GDI is released into the cytosol.
RabGDI deficiency and diseases[7] [8]
GDI1, one of the genes involved in the control of cycling between active and inactive state of the Rab family, has a major role in mental disorder. In fact, Gdi1 encodes α-GDI, which is specific for Rab3. Rab3 plays an important role in neurotransmitter release. Therefore, mutations in GDI1 cause functional and developmental alterations in the neuron which could lead to a severe impairment of learning abilities. More precisely, mutations in GDI1 are linked to X-linked nonspecific mental retardation. Two kinds of alterations in the GDI1 gene could be found and they determine two families affected with X-linked non-specific mental retardation (MRX48 and MRX41). In the family MRX48, there is a C -> T transition at position 366. The mutation disrupts synthesis of alpha-GDI by introducing a premature stop codon in the open reading frame. The mutation has a dominant phenotypic effect as carrier females in the family are also affected. The second mutation, in the MRX41 family, is a T -> C transition at position 433, causing a missense mutation and a non-conservative amino acid change (L92P). This mutation leads to a 6,3-fold decrease in affinity for Rab3A. The mutant α-GDI may not be able to efficiently recycle Rab proteins in vivo. The introduction of the helix-breaking proline residue at position 92 may either indirectly destabilize the adjacent Rab binding region or reduce the ability of alpha-GDI to recognize the C-terminus prenyl group required for high affinity binding during recycling. The major effect of both mutations could lead to a decrease of the Rab pool available for synaptic vesicles cycling and neurotransmitter release.
External resources
References
- ↑ Ignatev A, Kravchenko S, Rak A, Goody RS, Pylypenko O. A structural model of the GDP dissociation inhibitor rab membrane extraction mechanism. J Biol Chem. 2008 Jun 27;283(26):18377-84. Epub 2008 Apr 20. PMID:18426803 doi:10.1074/jbc.M709718200
- ↑ An Y, Shao Y, Alory C, Matteson J, Sakisaka T, Chen W, Gibbs RA, Wilson IA, Balch WE. Geranylgeranyl switching regulates GDI-Rab GTPase recycling. Structure. 2003 Mar;11(3):347-57. PMID:12623022
- ↑ Goody RS, Rak A, Alexandrov K. The structural and mechanistic basis for recycling of Rab proteins between membrane compartments. Cell Mol Life Sci. 2005 Aug;62(15):1657-70. PMID:15924270 doi:10.1007/s00018-005-4486-8
- ↑ Calero M, Chen CZ, Zhu W, Winand N, Havas KA, Gilbert PM, Burd CG, Collins RN. Dual prenylation is required for Rab protein localization and function. Mol Biol Cell. 2003 May;14(5):1852-67. Epub 2003 Feb 6. PMID:12802060 doi:10.1091/mbc.E02-11-0707
- ↑ Ignatev A, Kravchenko S, Rak A, Goody RS, Pylypenko O. A structural model of the GDP dissociation inhibitor rab membrane extraction mechanism. J Biol Chem. 2008 Jun 27;283(26):18377-84. Epub 2008 Apr 20. PMID:18426803 doi:10.1074/jbc.M709718200
- ↑ Pylypenko O, Rak A, Durek T, Kushnir S, Dursina BE, Thomae NH, Constantinescu AT, Brunsveld L, Watzke A, Waldmann H, Goody RS, Alexandrov K. Structure of doubly prenylated Ypt1:GDI complex and the mechanism of GDI-mediated Rab recycling. EMBO J. 2006 Jan 11;25(1):13-23. Epub 2006 Jan 5. PMID:16395334
- ↑ Dell'Amico MC, Vivani P, Miccoli M, Cecconi M, Baggiani A. Mutations in GDI1 and X-linked non-specific mental retardation. Ann Ig. 2011 Jan-Feb;23(1):71-9. PMID:21736009
- ↑ An Y, Shao Y, Alory C, Matteson J, Sakisaka T, Chen W, Gibbs RA, Wilson IA, Balch WE. Geranylgeranyl switching regulates GDI-Rab GTPase recycling. Structure. 2003 Mar;11(3):347-57. PMID:12623022