Papain
From Proteopedia
| Line 1: | Line 1: | ||
| - | <StructureSection load='9pap' size=' | + | <StructureSection load='9pap' size='350' side='left' caption='Papain [[9PAP]]' scene='Papain/Primary_scene/2' > |
| - | + | ||
| - | + | ||
This protease belongs to an extended family of aminopeptidases, dipeptidyl peptidases, endopeptidases, and other enzymes having both exo- and endo-peptidase activity. The inactivated zymogen with N-terminal propeptide regions - providing stability in alkaline environments and enabling proper folding - is activated through removal of the propeptide regions. <ref>PMID: 7845226</ref> The protein is primarily secreted with its pro-region enabling transport from zymogen to lysosome through membrane association and mediation. <ref>PMID: 12188906</ref> | This protease belongs to an extended family of aminopeptidases, dipeptidyl peptidases, endopeptidases, and other enzymes having both exo- and endo-peptidase activity. The inactivated zymogen with N-terminal propeptide regions - providing stability in alkaline environments and enabling proper folding - is activated through removal of the propeptide regions. <ref>PMID: 7845226</ref> The protein is primarily secreted with its pro-region enabling transport from zymogen to lysosome through membrane association and mediation. <ref>PMID: 12188906</ref> | ||
| Line 12: | Line 10: | ||
Papain's enzymatic use was first discovered in 1873 by G.C. Roy who published his results in the Calcutta Medical Journal in the article, "The Solvent Action of Papaya Juice on Nitrogenous Articles of Food." In 1879, papain was named officially by Wurtz and Bouchut, who managed to partially purify the product from the sap of papaya. It wasn't until the mid-twentieth century that the complete purification and isolation of papain was achieved. In 1968, Drenth et al. determined the structure of papain by x-ray crystallography, making it the second enzyme whose structure was successfully determined by x-ray crystallography. Additionally, papain was the first cysteine protease to have its structure identified.<ref name="Worthington" /> In 1984, Kamphuis et al. determined the geometry of the active site, and the three-dimensional structure was visualized to a 1.65 Angstrom solution.<ref name="Structure">PMID:6502713</ref> Today, studies continue on the stability of papain, involving changes in environmental conditions as well as testing of inhibitors such as phenylmethanesulfonylfluoride (PMSF), TLCK, TPCK, aplh2-macroglobulin, heavy metals, AEBSF, antipain, cystatin, E-64, leupeptin, sulfhydryl binding agents, carbonyl reagents, and alkylating agents.<ref name="Worthington" /> | Papain's enzymatic use was first discovered in 1873 by G.C. Roy who published his results in the Calcutta Medical Journal in the article, "The Solvent Action of Papaya Juice on Nitrogenous Articles of Food." In 1879, papain was named officially by Wurtz and Bouchut, who managed to partially purify the product from the sap of papaya. It wasn't until the mid-twentieth century that the complete purification and isolation of papain was achieved. In 1968, Drenth et al. determined the structure of papain by x-ray crystallography, making it the second enzyme whose structure was successfully determined by x-ray crystallography. Additionally, papain was the first cysteine protease to have its structure identified.<ref name="Worthington" /> In 1984, Kamphuis et al. determined the geometry of the active site, and the three-dimensional structure was visualized to a 1.65 Angstrom solution.<ref name="Structure">PMID:6502713</ref> Today, studies continue on the stability of papain, involving changes in environmental conditions as well as testing of inhibitors such as phenylmethanesulfonylfluoride (PMSF), TLCK, TPCK, aplh2-macroglobulin, heavy metals, AEBSF, antipain, cystatin, E-64, leupeptin, sulfhydryl binding agents, carbonyl reagents, and alkylating agents.<ref name="Worthington" /> | ||
| + | |||
| + | </StructureSection> | ||
| + | ---- | ||
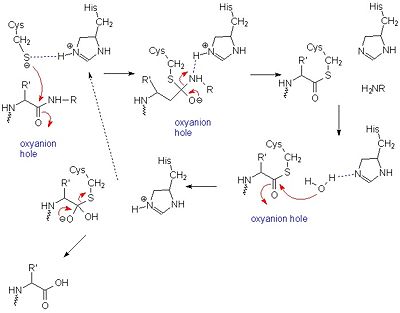
==Structure== | ==Structure== | ||
| Line 36: | Line 37: | ||
==Common Uses== | ==Common Uses== | ||
| - | + | ||
===Medicinal=== | ===Medicinal=== | ||
| + | [[Image:papayas.jpg|150px|right|thumb|Papaya<ref>http://dailyfitnessmagz.com/2011/03/papayas-nutrition-facts/</ref>]] | ||
Papain has been used for a plethora of medicinal purposes including treating inflammation, shingles, diarrhea, psoriasis, parasites, and many others.<ref>http://www.webmd.com/vitamins-supplements/ingredientmono-69-PAPAIN.aspx?activeIngredientId=69&activeIngredientName=PAPAIN</ref> One major use is the treatment of cutaneous ulcers including diabetic ulcers and pressure ulcers.<ref>http://www.pbm.va.gov/Clinical%20Guidance/Drug%20Monographs/Papain%20Urea.pdf</ref> Pressures ulcers plague many bed bound individuals and are a major source of pain and discomfort. Two papain based topical drugs are Accuzyme and Panafil, which can be used to treat wounds like cutaneous ulcers.<ref>http://www.pbm.va.gov/Clinical%20Guidance/Drug%20Monographs/Papain%20Urea.pdf</ref> | Papain has been used for a plethora of medicinal purposes including treating inflammation, shingles, diarrhea, psoriasis, parasites, and many others.<ref>http://www.webmd.com/vitamins-supplements/ingredientmono-69-PAPAIN.aspx?activeIngredientId=69&activeIngredientName=PAPAIN</ref> One major use is the treatment of cutaneous ulcers including diabetic ulcers and pressure ulcers.<ref>http://www.pbm.va.gov/Clinical%20Guidance/Drug%20Monographs/Papain%20Urea.pdf</ref> Pressures ulcers plague many bed bound individuals and are a major source of pain and discomfort. Two papain based topical drugs are Accuzyme and Panafil, which can be used to treat wounds like cutaneous ulcers.<ref>http://www.pbm.va.gov/Clinical%20Guidance/Drug%20Monographs/Papain%20Urea.pdf</ref> | ||
A recent New York Times article featured papain and other digestive enzymes.<ref>http://www.nytimes.com/2012/02/23/fashion/enzymes-once-sidelined-try-to-grab-the-spotlight.html</ref> With the number of individuals suffering from irritable bowel syndrome and other gastrointestinal issues, many people are turning toward natural digestive aid supplements like papain. The author even talks about the use of papain along with a pineapple enzyme, bromelain, in cosmetic facial masks. Dr. Adam R. Kolker (a plastic surgeon) is quoted in the article saying that "For skin that is sensitive, enzymes are wonderful." He bases these claims off the idea that proteases like papain help to break peptide bonds holding dead skin cells to the live skin cells.<ref>http://www.nytimes.com/2012/02/23/fashion/enzymes-once-sidelined-try-to-grab-the-spotlight.html</ref> | A recent New York Times article featured papain and other digestive enzymes.<ref>http://www.nytimes.com/2012/02/23/fashion/enzymes-once-sidelined-try-to-grab-the-spotlight.html</ref> With the number of individuals suffering from irritable bowel syndrome and other gastrointestinal issues, many people are turning toward natural digestive aid supplements like papain. The author even talks about the use of papain along with a pineapple enzyme, bromelain, in cosmetic facial masks. Dr. Adam R. Kolker (a plastic surgeon) is quoted in the article saying that "For skin that is sensitive, enzymes are wonderful." He bases these claims off the idea that proteases like papain help to break peptide bonds holding dead skin cells to the live skin cells.<ref>http://www.nytimes.com/2012/02/23/fashion/enzymes-once-sidelined-try-to-grab-the-spotlight.html</ref> | ||
| - | [[Image:2fab_fc.png|150px|right|thumb|Diagram showing cleavage products of an antibody digested by papain.]] | ||
===Commercial and Biomedical=== | ===Commercial and Biomedical=== | ||
Papain digests most proteins, often more extensively than pancreatic proteases. It has a very broad specificity and is known to cleave peptide bonds of basic amino acids and leucine and glycine residues, but prefers amino acids with large hydrophobic side chains. This non-specific nature of papain's hydrolase activity has led to its use in many and varied commercial products. It is often used as a meat tenderizer because it can hydrolyze the peptide bonds of collagen, elastin, and actomyosin. It is also used in contact lens solution to remove protein deposits on the lenses and marketed as a digestive supplement. <ref> http://www.webmd.com/vitamins-supplements/ingredientmono-69-PAPAIN.aspx?activeIngredientId=69&activeIngredientName=PAPAIN </ref> Finally, papain has several common uses in general biomedical research, including a gentle cell isolation agent, production of glycopeptides from purified proteoglycans, and solubilization of integral membrane proteins. It is also notable for its ability to specifically cleave IgG and IgM antibodies above and below the disulfide bonds that join the heavy chains and that is found between the light chain and heavy chain. This generates two monovalent Fab segments, that each have a single antibody binding sites, and an intact Fc fragment, as shown in the image to the right:<ref name="Worthington" /> | Papain digests most proteins, often more extensively than pancreatic proteases. It has a very broad specificity and is known to cleave peptide bonds of basic amino acids and leucine and glycine residues, but prefers amino acids with large hydrophobic side chains. This non-specific nature of papain's hydrolase activity has led to its use in many and varied commercial products. It is often used as a meat tenderizer because it can hydrolyze the peptide bonds of collagen, elastin, and actomyosin. It is also used in contact lens solution to remove protein deposits on the lenses and marketed as a digestive supplement. <ref> http://www.webmd.com/vitamins-supplements/ingredientmono-69-PAPAIN.aspx?activeIngredientId=69&activeIngredientName=PAPAIN </ref> Finally, papain has several common uses in general biomedical research, including a gentle cell isolation agent, production of glycopeptides from purified proteoglycans, and solubilization of integral membrane proteins. It is also notable for its ability to specifically cleave IgG and IgM antibodies above and below the disulfide bonds that join the heavy chains and that is found between the light chain and heavy chain. This generates two monovalent Fab segments, that each have a single antibody binding sites, and an intact Fc fragment, as shown in the image to the right:<ref name="Worthington" /> | ||
Revision as of 23:32, 3 April 2012
| |||||||||||
Contents |
Structure
| |||||||||||
Common Uses
Medicinal

Papain has been used for a plethora of medicinal purposes including treating inflammation, shingles, diarrhea, psoriasis, parasites, and many others.[22] One major use is the treatment of cutaneous ulcers including diabetic ulcers and pressure ulcers.[23] Pressures ulcers plague many bed bound individuals and are a major source of pain and discomfort. Two papain based topical drugs are Accuzyme and Panafil, which can be used to treat wounds like cutaneous ulcers.[24]
A recent New York Times article featured papain and other digestive enzymes.[25] With the number of individuals suffering from irritable bowel syndrome and other gastrointestinal issues, many people are turning toward natural digestive aid supplements like papain. The author even talks about the use of papain along with a pineapple enzyme, bromelain, in cosmetic facial masks. Dr. Adam R. Kolker (a plastic surgeon) is quoted in the article saying that "For skin that is sensitive, enzymes are wonderful." He bases these claims off the idea that proteases like papain help to break peptide bonds holding dead skin cells to the live skin cells.[26]
Commercial and Biomedical
Papain digests most proteins, often more extensively than pancreatic proteases. It has a very broad specificity and is known to cleave peptide bonds of basic amino acids and leucine and glycine residues, but prefers amino acids with large hydrophobic side chains. This non-specific nature of papain's hydrolase activity has led to its use in many and varied commercial products. It is often used as a meat tenderizer because it can hydrolyze the peptide bonds of collagen, elastin, and actomyosin. It is also used in contact lens solution to remove protein deposits on the lenses and marketed as a digestive supplement. [27] Finally, papain has several common uses in general biomedical research, including a gentle cell isolation agent, production of glycopeptides from purified proteoglycans, and solubilization of integral membrane proteins. It is also notable for its ability to specifically cleave IgG and IgM antibodies above and below the disulfide bonds that join the heavy chains and that is found between the light chain and heavy chain. This generates two monovalent Fab segments, that each have a single antibody binding sites, and an intact Fc fragment, as shown in the image to the right:[11]
Reference
- Kamphuis IG, Kalk KH, Swarte MB, Drenth J. Structure of papain refined at 1.65 A resolution. J Mol Biol. 1984 Oct 25;179(2):233-56. PMID:6502713
Page seeded by OCA on Tue Feb 17 04:20:31 2009
Proteopedia Page Contributors and Editors (what is this?)
Kirsten Eldredge, Jacinth Koh, Sara Kongkatong, Kyle Burch, Michal Harel, Joel L. Sussman, Elizabeth Miller, Samuel Bray, David Canner, Jaime Prilusky