User:Jamie Abbott/Sandbox2
From Proteopedia
(→Substrate Assisted Catalysis) |
(→Substrate Assisted Catalysis) |
||
| Line 30: | Line 30: | ||
The mechanism for transfer of the aminoacyl moiety from the aminoacyl-adenylate, generated in the adenylation reaction, onto it’s cognate tRNA is most easily explained by '''substrate assisted catalysis (SAC)'''. Substrate assisted catalysis preformed by HisRS is described as the occurrence of bond formation between the 3’OH of tRNAHis and the α-carboxylate carbon of the aminoacyl adenylate prior to the cleavage of the bond joining the α-carboxylate carbon to the axial oxygen of the α-phosphate. This concerted SAC mechanism, rationalizes the considerable pKa difference between the 3’OH of tRNA (pKa =16) and the nonbridging Sp oxygen (pKa= -1)<ref name="GUTH05" />. Therefore, as the bond between the tRNAHis nucleophile and the α-carboxylate is formed, the pKa for the 3’OH would be expected to drop sharply and the pKa of the nonbridging oxygen would tend to rise as the bond to the α-carboxylate lengthens and breaks<ref name="GUTH05" />. | The mechanism for transfer of the aminoacyl moiety from the aminoacyl-adenylate, generated in the adenylation reaction, onto it’s cognate tRNA is most easily explained by '''substrate assisted catalysis (SAC)'''. Substrate assisted catalysis preformed by HisRS is described as the occurrence of bond formation between the 3’OH of tRNAHis and the α-carboxylate carbon of the aminoacyl adenylate prior to the cleavage of the bond joining the α-carboxylate carbon to the axial oxygen of the α-phosphate. This concerted SAC mechanism, rationalizes the considerable pKa difference between the 3’OH of tRNA (pKa =16) and the nonbridging Sp oxygen (pKa= -1)<ref name="GUTH05" />. Therefore, as the bond between the tRNAHis nucleophile and the α-carboxylate is formed, the pKa for the 3’OH would be expected to drop sharply and the pKa of the nonbridging oxygen would tend to rise as the bond to the α-carboxylate lengthens and breaks<ref name="GUTH05" />. | ||
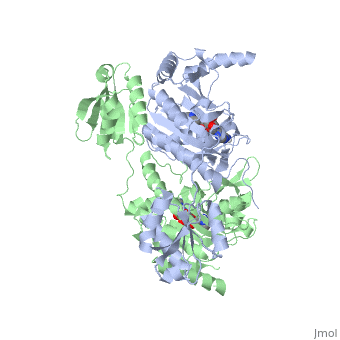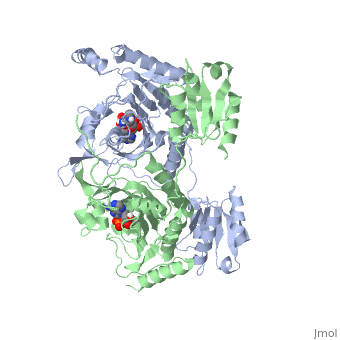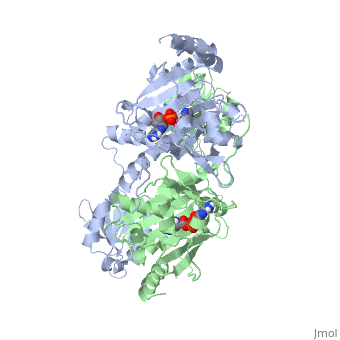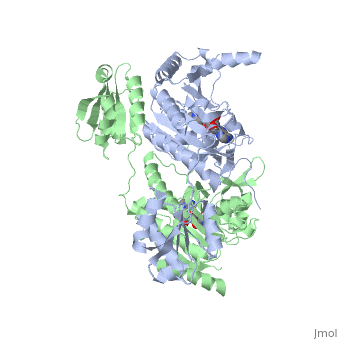
| - | [[Image:New ProRproS.jpg|thumb| | + | [[Image:New ProRproS.jpg|thumb|left|upright=1.0|550px|'''Substrate assisted mechanism catalyzed by histidyl-tRNA synthetase.''']] |
== Histidine tRNA Recognition == | == Histidine tRNA Recognition == | ||
Revision as of 16:32, 16 April 2012
Contents |
Histidyl-tRNA Synthetase
Histidyl tRNA Synthetase (HisRS) is a 94kD that belongs to the class II of aminoacyl-tRNA synthetases (aaRS). Aminoacyl-tRNA synthetases Aminoacyl-tRNA synthetases have been partitioned into two classes, containing 10 members, on the basis of sequence comparisons[1]. Class I and Class II differ mainly with respect to the topology of the catalytic fold and site of esterification on cognate tRNA[1]. Class II enzymes have a composed of anti-parallel β-sheets and α-helices (residues 1-325). Additionally, class II enzymes can be further divided into three subgroups: class IIa, distinguished by an N-terminal catalytic domain and C-terminal accessory domain (later shown to be ); class IIb, whose anticodon binding domain is located on the N-terminal side of the fold; and class IIc, encompassing the tetrameric PheRS and GlyRS class II synthetases.[2]
| |||||||||||
Mechanism
Electrophilic Catalysis
The HisRS active site contains a highly conserved residue, Arg259, takes part in electrophilic catalysis for the adenylation reaction. First, as Arg259 is positioned on the HisA loop serves to fix the α-carboxylate group of the histidine substrate as the attacking nucleophile[6]. Second, the guanidinium group of Arg259 is positioned approximately 3Å from the α-phosphate of ATP where it serves as the electrophilic catalyst. Arg113 as well as Arg259 are arranged to interact with α-phosphate of ATP and thereby stabilize negative charge developed on the non-bridging oxygens during the transition state aarsbk. Evidence for Arg259 playing a critical role in catalysis is observed in a two or three log decrease in activity when substituted with a histidine [4] or other amino acids[7]. Arg259 also interacts with the phenolic OH of Tyr264, which in turn donates a hydrogen bond to the Nδ of the histidine substrate[3]. Utilizing Arg259 for catalysis is unique to HisRS as other class II aaRS enzymes, AspRS[8] and SerRS[9], use a divalent magnesium metal ion to coordinate the α-phosphate of ATP and serve as an electrophilic catalysis.
Substrate Assisted Catalysis
The second reaction carried out by HisRS, aminoacylation, requires the decomposition of a mixed anhydride (the ) to form an aminoacyl ester on the 3’OH of tRNAHis. It was initially hypothesized that Glu83, acting as a general base, would improve the rate of this reaction. However, while Glu83 is in a favorable position in the active site to function as a base it is also situated to neutralize the α-amino group of the histidine substrate. Thus, mutational analysis of Glu83[10] suggests that it does not act as a base but forms a salt bridge with the α-amino group of histidine, neutralizing it’s charge, and satisfying a critical electrostatic interaction.
The mechanism for transfer of the aminoacyl moiety from the aminoacyl-adenylate, generated in the adenylation reaction, onto it’s cognate tRNA is most easily explained by substrate assisted catalysis (SAC). Substrate assisted catalysis preformed by HisRS is described as the occurrence of bond formation between the 3’OH of tRNAHis and the α-carboxylate carbon of the aminoacyl adenylate prior to the cleavage of the bond joining the α-carboxylate carbon to the axial oxygen of the α-phosphate. This concerted SAC mechanism, rationalizes the considerable pKa difference between the 3’OH of tRNA (pKa =16) and the nonbridging Sp oxygen (pKa= -1)[10]. Therefore, as the bond between the tRNAHis nucleophile and the α-carboxylate is formed, the pKa for the 3’OH would be expected to drop sharply and the pKa of the nonbridging oxygen would tend to rise as the bond to the α-carboxylate lengthens and breaks[10].
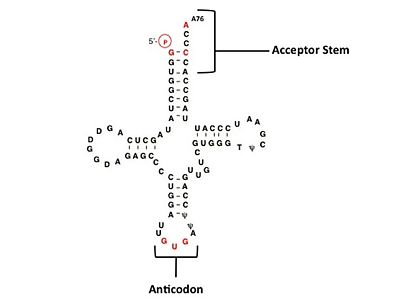
Histidine tRNA Recognition
The accuracy of protein synthesis is dependent upon the ability of aminoacyl-tRNA synthetases to specifically recognize the cognate tRNA and attach the appropriate amino acid. The identity nucleotides that define tRNA isoacceptor systems are primarily concentrated in the anticodons and acceptor stems of tRNAs, providing functional groups that can be accessed by specificity-determining side chains on the enzymes (8422978, 1857417). Although, tRNA identity can also emerge from the presence of modified bases (3054566). Key identity elements on E. coli histidine tRNA include the 5’ phosphate, G-1:C73 base pair in the acceptor stem and the GUG anticodon. Mutations of these identity elements diminishes aminoacylation in vitro[11][12][13]. Residues throught to be involved in the recognition of these identity elements include; Arg123, Arg116, and Gln118. Substitution in Arg123 as a putative contact to the 5’phosphate, produced a 200 fold decrease in aminoacyl-transfer[14]. Similar kinetic defects in aminoacyl-transfer were also observed for Arg116 and Gln118 [14].
Evolutionary Conservation
Structural Homology
3D Structures of Histidyl-tRNA Synthetase
Bacteria
Eukaryota
Archara
References
- ↑ 1.0 1.1 Eriani G, Delarue M, Poch O, Gangloff J, Moras D. Partition of tRNA synthetases into two classes based on mutually exclusive sets of sequence motifs. Nature. 1990 Sep 13;347(6289):203-6. PMID:2203971 doi:http://dx.doi.org/10.1038/347203a0
- ↑ Cusack S, Hartlein M, Leberman R. Sequence, structural and evolutionary relationships between class 2 aminoacyl-tRNA synthetases. Nucleic Acids Res. 1991 Jul 11;19(13):3489-98. PMID:1852601
- ↑ 3.0 3.1 3.2 3.3 Francklyn, C., and Arnez, J.G. (2004) in Aminoacyl-tRNA Synthetases (Ibba, M.,Francklyn, C.,Cusack, S.. Eds.) Landes Publishing, Austin, TX
- ↑ 4.0 4.1 Arnez JG, Augustine JG, Moras D, Francklyn CS. The first step of aminoacylation at the atomic level in histidyl-tRNA synthetase. Proc Natl Acad Sci U S A. 1997 Jul 8;94(14):7144-9. PMID:9207058
- ↑ Arnez JG, Moras D. Structural and functional considerations of the aminoacylation reaction. Trends Biochem Sci. 1997 Jun;22(6):211-6. PMID:9204708
- ↑ Arnez JG, Flanagan K, Moras D, Simonson T. Engineering an Mg2+ site to replace a structurally conserved arginine in the catalytic center of histidyl-tRNA synthetase by computer experiments. Proteins. 1998 Aug 15;32(3):362-80. PMID:9715912
- ↑ Ruhlmann A, Cramer F, Englisch U. Isolation and analysis of mutated histidyl-tRNA synthetases from Escherichia coli. Biochem Biophys Res Commun. 1997 Aug 8;237(1):192-201. PMID:9266856 doi:10.1006/bbrc.1997.7108
- ↑ Poterszman A, Delarue M, Thierry JC, Moras D. Synthesis and recognition of aspartyl-adenylate by Thermus thermophilus aspartyl-tRNA synthetase. J Mol Biol. 1994 Nov 25;244(2):158-67. PMID:7966328 doi:http://dx.doi.org/10.1006/jmbi.1994.1716
- ↑ Belrhali H, Yaremchuk A, Tukalo M, Berthet-Colominas C, Rasmussen B, Bosecke P, Diat O, Cusack S. The structural basis for seryl-adenylate and Ap4A synthesis by seryl-tRNA synthetase. Structure. 1995 Apr 15;3(4):341-52. PMID:7613865
- ↑ 10.0 10.1 10.2 Guth E, Connolly SH, Bovee M, Francklyn CS. A substrate-assisted concerted mechanism for aminoacylation by a class II aminoacyl-tRNA synthetase. Biochemistry. 2005 Mar 15;44(10):3785-94. PMID:15751955 doi:10.1021/bi047923h
- ↑ Fromant M, Plateau P, Blanquet S. Function of the extra 5'-phosphate carried by histidine tRNA. Biochemistry. 2000 Apr 11;39(14):4062-7. PMID:10747795
- ↑ Himeno H, Hasegawa T, Ueda T, Watanabe K, Miura K, Shimizu M. Role of the extra G-C pair at the end of the acceptor stem of tRNA(His) in aminoacylation. Nucleic Acids Res. 1989 Oct 11;17(19):7855-63. PMID:2678006
- ↑ Yan W, Francklyn C. tRNA selection by a class II aminoacyl-tRNA synthetase: the role of accessory domains and inter-domain communication in RNA recognition. Nucleic Acids Symp Ser. 1995;(33):167-9. PMID:8643360
- ↑ 14.0 14.1 Guth EC, Francklyn CS. Kinetic discrimination of tRNA identity by the conserved motif 2 loop of a class II aminoacyl-tRNA synthetase. Mol Cell. 2007 Feb 23;25(4):531-42. PMID:17317626 doi:10.1016/j.molcel.2007.01.015