User:Marvin O'Neal/OspC
From Proteopedia
(→'''Outer Surface Protein (OspC)''') |
(→'''Introduction''') |
||
| Line 6: | Line 6: | ||
=='''Introduction'''== | =='''Introduction'''== | ||
| - | Lyme disease is a typical epidemic in northeastern and north central regions of the United States. It is also the most prevalent tick-borne disease which is caused by ''Borrelia burgdoferi'',a spirochete that is identified in ''Ixodes scapulars''. Humans infected with Lyme disease begin with unusal skin lesions (erythema chronicum migrans), which later develop into more severe neurological and cardiac symptoms. If it is not completely treated, the causative agent of Lyme disease persists in human by interacting the surface-exposed | + | Lyme disease is a typical epidemic in northeastern and north central regions of the United States. It is also the most prevalent tick-borne disease which is caused by ''Borrelia burgdoferi'',a spirochete that is identified in ''Ixodes scapulars''. Humans infected with Lyme disease begin with unusal skin lesions (erythema chronicum migrans), which later develop into more severe neurological and cardiac symptoms. If it is not completely treated, the causative agent of Lyme disease persists in human by interacting the surface-exposed ''B. burgdoferi'' proteins with the molecules of the host. Among 100 different lipoproteins present in its outer surface, the outer surface protein C (OspC) is one of its major host-induced antigens, but is inactive due to the immune response of mammalian host during its early age. The expression of OspC is dependent on temperature and it is induced when the tick migrates from guts to salivary gland during feeding period. OspC is highly polymorphic and its genetic diversity at the OspC locus can be categorized into 19 outer surface protein major groups (oMGs) ranged from type A to S based on alignment sequencing. However, only four invasive allelic groups: A, B, I, and K, are responsible for causing human Lyme disease. |
=='''Ecology of Lyme Disease'''== | =='''Ecology of Lyme Disease'''== | ||
Revision as of 20:35, 27 April 2012
Hello!
|
This is the which we came out after reading the primary literature.
Contents |
Introduction
Lyme disease is a typical epidemic in northeastern and north central regions of the United States. It is also the most prevalent tick-borne disease which is caused by Borrelia burgdoferi,a spirochete that is identified in Ixodes scapulars. Humans infected with Lyme disease begin with unusal skin lesions (erythema chronicum migrans), which later develop into more severe neurological and cardiac symptoms. If it is not completely treated, the causative agent of Lyme disease persists in human by interacting the surface-exposed B. burgdoferi proteins with the molecules of the host. Among 100 different lipoproteins present in its outer surface, the outer surface protein C (OspC) is one of its major host-induced antigens, but is inactive due to the immune response of mammalian host during its early age. The expression of OspC is dependent on temperature and it is induced when the tick migrates from guts to salivary gland during feeding period. OspC is highly polymorphic and its genetic diversity at the OspC locus can be categorized into 19 outer surface protein major groups (oMGs) ranged from type A to S based on alignment sequencing. However, only four invasive allelic groups: A, B, I, and K, are responsible for causing human Lyme disease.
Ecology of Lyme Disease
Ecological factors responsible for Human Lyme disease risk
Dilution Effect Model
Outer Surface Protein (OspC)
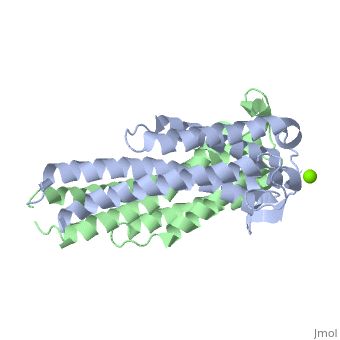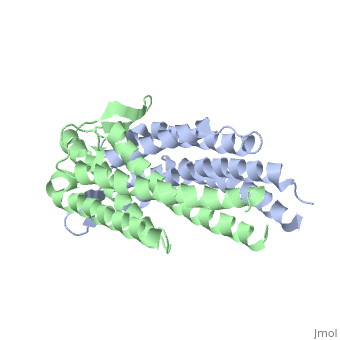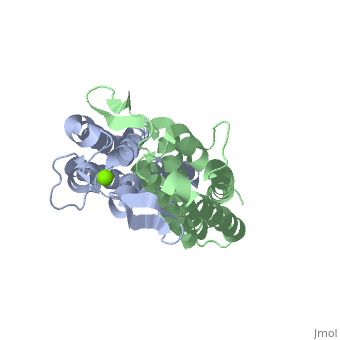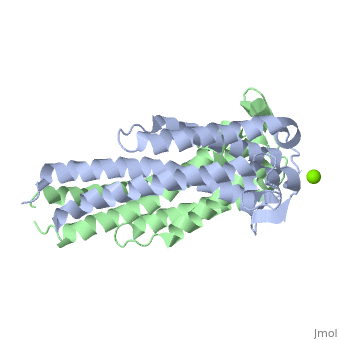
The model presented is B31 strain (residues 38-201), which is also known as oMG A. This is one of four invasive oMGs that are responsible for systematic Lyme disease. In crystal structure, OspC exists as a with the coordination of divalent ion, which is modeled as a magnesium ion. Each subunit is predominantly helical, consisting of five parallel , two short antiparallel and six . Since random coils are the most antigenic sites on the protein, pink and red colors were applied to protein in order to differentiate the polymorphism among these major antigenic sites (Earnhart 2010).The more polymorphic random coil on the protein model, residue 146 to 150 and residue 77 to 93, are colored in red. Two short β sheets are included in this region. On the other hand, two least polymorphic random coils, residue 115 to 119 and residue 161 to 169, are colored in pink.