User:Jamie Abbott/Sandbox2
From Proteopedia
(→Mechanism) |
(→Mechanism) |
||
| Line 31: | Line 31: | ||
| [[Image:Aminoacylation.jpg|center|600px|'''Aminoacylation reaction catalyzed by HisRS.''']] | | [[Image:Aminoacylation.jpg|center|600px|'''Aminoacylation reaction catalyzed by HisRS.''']] | ||
|} | |} | ||
| - | |||
| - | |||
=== Electrophilic Catalysis === | === Electrophilic Catalysis === | ||
Revision as of 15:53, 29 April 2012
Contents |
Histidyl-tRNA Synthetase
Histidyl tRNA Synthetase (HisRS) is a 94kD that belongs to the class II of aminoacyl-tRNA synthetases (aaRS). Aminoacyl-tRNA synthetases are classified as ligases where as they require a high energy ATP cofactor to ligate a specific amino acid to their cognate tRNAs. Histidyl-tRNA synthetase more specifically attaches amino acid histidine to tRNAHis. Histidine is a unique amino acid often part of enzyme active sites where it can act as either a base or an acid during acid-base catalysis. Aminoacyl-tRNA synthetases have been partitioned into two classes, containing 10 members, on the basis of sequence comparisons[1]. Class I and Class II differ mainly with respect to the topology of the catalytic fold and site of esterification on cognate tRNA[1]. Class II enzymes have a composed of anti-parallel β-sheets and α-helices (residues 1-325). Additionally, class II enzymes can be further divided into three subgroups: class IIa, distinguished by an N-terminal catalytic domain and C-terminal accessory domain (later shown to be ); class IIb, whose anticodon binding domain is located on the N-terminal side of the fold; and class IIc, encompassing the tetrameric PheRS and GlyRS class II synthetases.[2]
Substrate Specificity
| |||||||||||
Mechanism
Electrophilic Catalysis
The HisRS active site contains a highly conserved residue in the HisRS family, Arg259, that takes part in electrophilic catalysis for the adenylation reaction. This active site arginine residue is not present in other aaRS class II enzymes. As noted in the above discussion about metal ion coordination Arg259 and Arg113 are positioned to interact with the α-phosphate of ATP to assist in the adenylation reaction. First, as Arg259 is positioned on the HisA loop serves to fix the α-carboxylate group of the histidine substrate as the [8]. Second, one ηN of the guanidinium group of Arg259 is positioned approximately 3Å from the α-phosphate of ATP while the other ηN hydrogen bonds with phenolic group of Tyr264. Furthermore, the Tyr264 residue is then stabilized to also hydrogen bond to the Nδ of the histidine substrate[3].
A comparison of Glu270 in the HisRS:histidinol and the HisRS:adenylate complexes provides further structural information into how Arg259 may serve a role in catalysis. In the HisRS:histidinol complex a water-mediated interaction between Glu270 and εN of Arg259. However, in the HisRS:adenylate complex Glu270 moves to form a salt bridge with the guanidinium group and to exclude the water molecule. This movement serves as a salt bridge switch that may weaken the ionic interaction between Arg259 and the α-phosphate(arnez1st step) and stabilize adenylate formation in the active site. Also, Arg113 as well as Arg259 are arranged to interact with of ATP and and also stabilize negative charge developed on the non-bridging oxygens α-phosphate during the transition state [3]. Evidence for Arg259 playing a critical role in catalysis is further supported by mutational studies where a two or three log decrease in activity is observed when Arg259 is substituted with a histidine [4] or other amino acids[9]. Utilizing Arg259 for catalysis is unique to HisRS as other class II aaRS enzymes, AspRS[10] and SerRS[7], use a divalent magnesium metal ion to coordinate the α-phosphate of ATP and serve as an electrophilic catalyst.
Substrate Assisted Catalysis
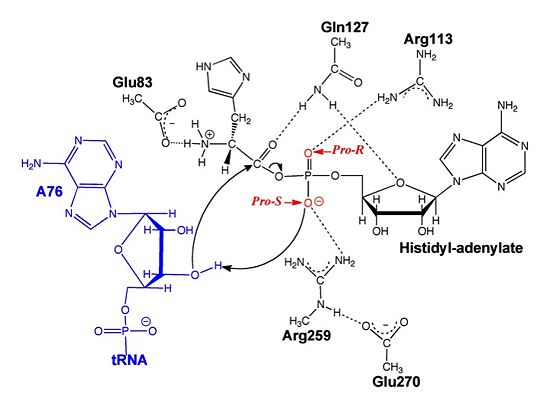
The second reaction carried out by HisRS, aminoacylation, requires the decomposition of a mixed anhydride (the ) to form an aminoacyl ester on the 3’OH of tRNAHis. It was initially hypothesized that Glu83, acting as a general base, would improve the rate of this reaction. However, while Glu83 is in a favorable position in the active site to function as a base it is also situated to neutralize the α-amino group of the histidine substrate. Thus, mutational analysis of Glu83[11] suggests that it does not act as a base but forms a salt bridge with the α-amino group of histidine, neutralizing it’s charge, and satisfying a critical electrostatic interaction.
The mechanism for transfer of the aminoacyl moiety from the aminoacyl-adenylate, generated in the adenylation reaction, onto it’s cognate tRNA is most easily explained by substrate assisted catalysis (SAC). Substrate assisted catalysis preformed by HisRS is described as the occurrence of bond formation between the 3’OH of tRNAHis and the α-carboxylate carbon of the aminoacyl adenylate prior to the cleavage of the bond joining the α-carboxylate carbon to the axial oxygen of the α-phosphate. This concerted SAC mechanism, rationalizes the considerable pKa difference between the 3’OH of tRNA (pKa =16) and the nonbridging Sp oxygen (pKa= -1)[11]. Therefore, as the bond between the tRNAHis nucleophile and the α-carboxylate is formed, the pKa for the 3’OH would be expected to drop sharply and the pKa of the nonbridging oxygen would tend to rise as the bond to the α-carboxylate lengthens and breaks[11].