Outer surface protein A) is an abundant lipoprotein of the causative agent of Lyme disease, Borrelia burgdorferi spirochete. Its purpose is to stimulate the production of specific antibodies against B. burgdorferi and is used as a vaccination against Lyme disease, the disease carried by ticks("DrugBank: 2012).
It is a borrelial protein that colonizes within the transmitting tick host midgut. Lyme disease is a progressive infection resulting from inoculation of the spirochete B. burgdorferi into the skin by a feeding tick, usually species Ixodes ("Crystal Structure 2012). If left untreated, it may progress in many stages beginning from minor symptoms like rash, commonly seen as a bull-eyes rash and then progress into serious, lifelong disabilities. One of the autoimmune processes that trigger an inflammatory response occurs from the endurance of borrelia in the host. It is known to cause demyelinating diseases in the Central Nervous System (CNS). Once the borrelia is in the CNS, OspA is up regulated for the sake of adhesion to the area (Durovska 2011). Initially, OspA is down regulated upon infection of the host but it is up regulated again in the different envirnments of the host of the Cerebral Spinal Fluid (CSF). It is hard for our immune system to attack the borrelia because there is an inactivation of the effector mechanisms and also because of the borrelia hides in less accessible compartments in our body (Rupprecht 2008). The main mechanism of protection is the blockage of borrelia transmission from tick to host by anti OspA antibodies (de Silva 1996). However, further studies need to be done in order to improve the OspA based vaccines.
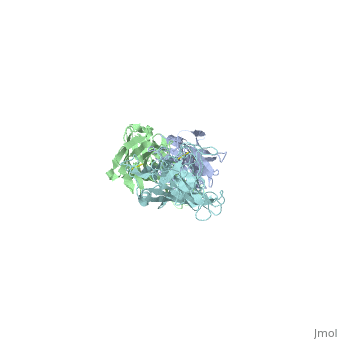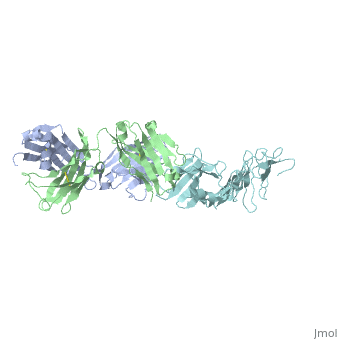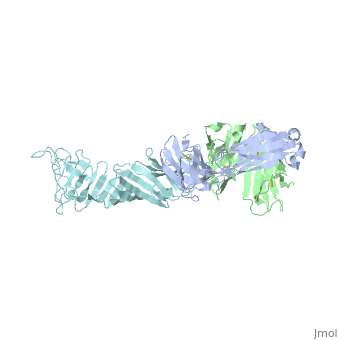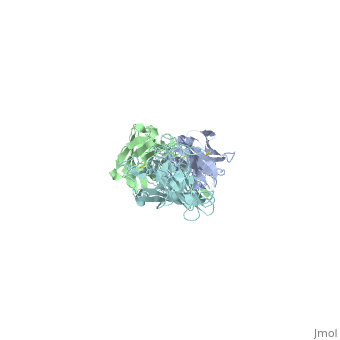
Here, a 3-D prototype was created to portray its structures. OspA is composed of a prolonged fold with 21 anti-parallel beta sheets and a single alpha helix. They are arranged to form the N-terminal, central sheet and C –terminal barrel domains OspA has numerous features, including a unique folding pattern that includes alternating charge arrays into antiparallel β-sheet, a potential ligand binding site, a conserved surface overlapping the epitope of the Fab, and a distinctive variable motif. It suggests that the protein has a conserved function, possibly acting as a receptor or signal transducer ("Crystal Structure 2012). Although OspA normally has a lipidated N-terminal cysteine to provide a membrane anchor (Brandt 1990), a recombinant unlipidated form is soluble in aqueous solution and is still recognized by antibodies from Lyme disease patients (Dunn 1990). It was identified that three loops of connecting beta strands are the primary sites of binding.
, colored red on the 3-D prototype, consists of residues 203 to 220. However, a natural variation found on colored yellow, of the first loop is a significant limiting factor in the antibody binding. , colored purple, consists of residues 224 to 233. And , colored blue, consists of residues 246 to 257. The remainder of the beta sheets was colored cyan because they did not play any significant role in OspA. This prototype suggests the importance of the three specific loops and their purpose to binding.
References
Brandt M E, Riley B S, Radolf J D, Norgard M V (1990) Infect Immun58:983–991
"Crystal Structure of Lyme Disease Antigen Outer Surface Protein A Complexed With an L-Fab." Crystal Structure of Lyme Disease Antigen Outer Surface Protein A Complexed With an L-Fab. Web. 20 Apr. 2012. <http://www.pnas.org/content/94/8/3584.full>.
de Silva A M, Telford S R III, Brunet L R, Barthold S W, Fikrig (1996) J Exp Med 183:271–275
"DrugBank: OspA Lipoprotein (DB00045)." DrugBank. Web. 20 Apr. 2012. <http://www.drugbank.ca/drugs/DB00045>.
Dunn J J, Lade B N, Barbour A G(1990) Protein Expression Purif 1:159–168
Durovska,J,S Bazovska, et al. “infection with B.burgdorferi s.l., and the CNS demyelinating disease. A case report.” PubMed. 32.4(2011):411-414. 20 Apr.2012. <pubmed.gov>.
Rupprecht, Tobias A. “The Pathogenesis of Lyme Neuroborreliosis: From Infection to Inflammation.” MOL MED. (2008): 205-212.