Sandbox Reserved 484
From Proteopedia
| Line 18: | Line 18: | ||
The protein Cathepsin B has many molecular functions. Some of these functions include endopeptidase activity, cysteine-type endopeptidase activity, protein binding, peptidase activity, cysteine-type peptidase activity, hydrolase activity, kininogen binding, and peptide binding. | The protein Cathepsin B has many molecular functions. Some of these functions include endopeptidase activity, cysteine-type endopeptidase activity, protein binding, peptidase activity, cysteine-type peptidase activity, hydrolase activity, kininogen binding, and peptide binding. | ||
== Mechanism of Action == | == Mechanism of Action == | ||
| + | [[Image:Alzheimers.JPG | thumb]] | ||
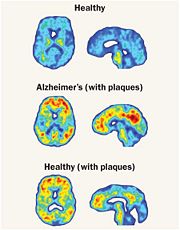
| + | One very crucial function of cathepsin B that I would like to emphasize on is its ability to break down the proteins that cause amyloid plaque, the root cause of Alzheimer’s systems. So, Cathepsin B has a built in protective mechanism against Alzheimer’s disease. Cathepsin B functions by “snipping” apart proteins which are closely associated with the amyloid protein that forms the amyloid plaques. Once, Cathepsin B breaks down these amyloid plaques, it signals other enzymes to further degrade the protein. An experiment was conducted in which a group of researchers found that cutting out the Cathepsin B gene increased the plaque deposition in mice with Alzheimers disease symptoms. The mice showed the human form of amyloid precursor protein (APP). These researchers also discovered that Cathepsin B piled up within the amyloid plaques and that it acted to decrease levels in neurons. The researchers also found that introducing a pathological form APP called APP1-42 into the neurons drastically increased cathepsin B in young and middle aged mice with human APP, but not old mice. From this observation, the researchers concluded that the upregulation of Cathepsin B could represent a protective mechanism that fails with aging and a failure like this could mean that it plays a role in late-onset sporadic Alzheimer’s disease. | ||
== Medical Implications or Possible Application == | == Medical Implications or Possible Application == | ||
== References == | == References == | ||
Revision as of 20:19, 1 May 2012
| This Sandbox is Reserved from 13/03/2012, through 01/06/2012 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 451 through Sandbox Reserved 500. | |||||||
To get started:
More help: Help:Editing For more help, look at this link: http://www.proteopedia.org/wiki/index.php/Help:Getting_Started_in_Proteopedia
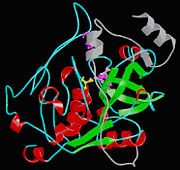
Cathepsin BCathepsin B is a very complex protein which is a part of the super family of papain-like cystein proteases. These papain-like cysteine proteases are abundant in nature and can be found in baculovirus, eubacteria, yeast, plants, and animals. They are synthesized as inactive proenzymeswith N-terminal pro-peptide regions. The pro-peptide plays an important role as an inhibitor of enzymatic activity. A number of cysteine proteases are located within lysosomes and they have very specific functions in endo and exopeptidase activity. Cathepsin B is synthesized as a pre-proenzyme of 339 amino acid residues and it can act as an endopeptidase. Its activity is inhibited by a2-macroglobulin . The most crucial functions of Cathepsin B are that it is implicated in the turnover of proteins and carries out different roles in maintaining the normal metabolism of cells. Cathepsin B is found in humans and other mammals and it is encoded by a single gene and this gene exhibits a high degree of sequence homology to other cysteine proteases of the papain super family. However, in parasitic helmiths and free-living nematodes, the Cathepsin B genes can be seen as large multigene families instead of just one single gene shown in humans and mammals. The function of this Cathepsin B gene has not been verified for sure, but research is showing that Cathepsin B could be involved in induction of protective immunity. In diseases, such as cancer and arthritis, Cathepsin B gene shows up regulation and in Alzheimer’s disease down regulation is shown.
Structure
Isolated from human liver, the protein Cathepsin B has a very unique structure. Human Cathepsin B contains 14 cysteine residues, with Cys29 representing the active site cysteine, and 12 other cysteine residues found to be involved in disulphide bridges. Also, Cathepsin B contains six dilsulfide bridges which are enclosed to the amino-terminal half of the molecule. Two of the cysteine residues (Cys 29 and Cys 240) are unpaired. The crystal structure of Cathepsin B consist of a double-chain form, with one light chain and one heavy chain composed of residues Leu1-Asn47 and Val50-Asp254. Some other features of Cathepsin B is that it has a diameter of 50 angstroms and a thickness of 30 angstroms. Also, you can see that there is a marked incision on the structure which represents the active site cleft. The polypeptide chain is described as being folded into two distinct domains which are known to interact with one another through an extended polar interface which opens to the V-shaped active site cleft. The left-hand” L” domain is formed by the amino-terminal half of the polypeptide chain up to about Tyr 148 and by the last carboxy-terminal residues. The right hand “R” domain is composed of the first ten residues and the carboxy-terminal half of the polypeptide chain. The amino and the carboxy-terminal ends act as ‘straps’ which help in clamping both domains together. FunctionThe protein Cathepsin B has many molecular functions. Some of these functions include endopeptidase activity, cysteine-type endopeptidase activity, protein binding, peptidase activity, cysteine-type peptidase activity, hydrolase activity, kininogen binding, and peptide binding. Mechanism of ActionOne very crucial function of cathepsin B that I would like to emphasize on is its ability to break down the proteins that cause amyloid plaque, the root cause of Alzheimer’s systems. So, Cathepsin B has a built in protective mechanism against Alzheimer’s disease. Cathepsin B functions by “snipping” apart proteins which are closely associated with the amyloid protein that forms the amyloid plaques. Once, Cathepsin B breaks down these amyloid plaques, it signals other enzymes to further degrade the protein. An experiment was conducted in which a group of researchers found that cutting out the Cathepsin B gene increased the plaque deposition in mice with Alzheimers disease symptoms. The mice showed the human form of amyloid precursor protein (APP). These researchers also discovered that Cathepsin B piled up within the amyloid plaques and that it acted to decrease levels in neurons. The researchers also found that introducing a pathological form APP called APP1-42 into the neurons drastically increased cathepsin B in young and middle aged mice with human APP, but not old mice. From this observation, the researchers concluded that the upregulation of Cathepsin B could represent a protective mechanism that fails with aging and a failure like this could mean that it plays a role in late-onset sporadic Alzheimer’s disease. Medical Implications or Possible ApplicationReferences |