We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 480
From Proteopedia
(Difference between revisions)
| Line 13: | Line 13: | ||
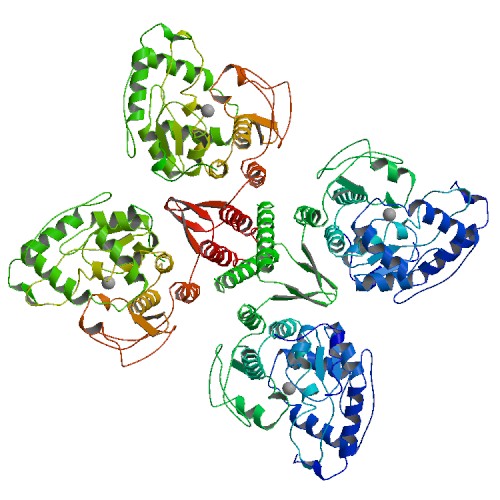
It has been observed as a <scene name='Sandbox_Reserved_480/Dimer/1'>dimer</scene> and tetrameric structure. This is the <scene name='Sandbox_Reserved_480/Active_site/3'>active</scene> site within the subunit. This includes the residues His, His, and | It has been observed as a <scene name='Sandbox_Reserved_480/Dimer/1'>dimer</scene> and tetrameric structure. This is the <scene name='Sandbox_Reserved_480/Active_site/3'>active</scene> site within the subunit. This includes the residues His, His, and | ||
This enzyme has two <scene name='Sandbox_Reserved_480/Ligands/1'>ligands</scene>, Fe (III) and . | This enzyme has two <scene name='Sandbox_Reserved_480/Ligands/1'>ligands</scene>, Fe (III) and . | ||
| - | |||
| - | == Mechanism == | ||
| - | |||
== Medical Implications == | == Medical Implications == | ||
| + | == Mechanism == | ||
== References == | == References == | ||
Revision as of 04:04, 2 May 2012
| This Sandbox is Reserved from 13/03/2012, through 01/06/2012 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 451 through Sandbox Reserved 500. | |||||||
To get started:
More help: Help:Editing For more help, look at this link: http://www.proteopedia.org/wiki/index.php/Help:Getting_Started_in_Proteopedia
Phenylalanine Hydroxylase
Phenylalanine Hydroxylase()is a type of enzyme involved in the catalization of phenylalanine into tyrosine. IntroductionStructureIt has been observed as a and tetrameric structure. This is the site within the subunit. This includes the residues His, His, and This enzyme has two , Fe (III) and . Medical ImplicationsMechanismReferences |