User:Marvin O'Neal/OspA
From Proteopedia
m |
m |
||
| Line 62: | Line 62: | ||
<h2>OspA Vaccination</h2> | <h2>OspA Vaccination</h2> | ||
<p> | <p> | ||
| - | Risk of developing Lyme disease can be <span style="color:red">mitigated</span> by staying clear of areas with populations of ticks, wearing proper attire <span style="color:red">to minimize easily bitten areas of the body</span>, and using <span style="color:red">insect repellents containing</span> [http://en.wikipedia.org/wiki/DEET DEET] (N,N-diethy-m-toluamide). However, <span style="color:red">another effective means for prevention could be possible</span> by using an outer surface protein from <i>Borrelia</i> <span style="color:red">in the development of</span> a vaccine | + | Risk of developing Lyme disease can be <span style="color:red">mitigated</span> by staying clear of areas with populations of ticks, wearing proper attire <span style="color:red">to minimize easily bitten areas of the body</span>, and using <span style="color:red">insect repellents containing</span> [http://en.wikipedia.org/wiki/DEET DEET] (N,N-diethy-m-toluamide). However, <span style="color:red">another effective means for prevention could be possible</span> by using an outer surface protein from <i>Borrelia</i> <span style="color:red">in the development of</span> a vaccine.<ref name="nigrovic">PMID: 16893489</ref> |
</p> | </p> | ||
<p> | <p> | ||
| - | <span style="color:red">The membrane composition of <i>Borrelia</i> is abundant in both OspA and OspB, and the two proteins share a 53% similarity in their primary sequences. Both are also expressed in the tick's gut and downregulated during feeding and aid in its survivability; however, OspA is overall less varied and reactive than OspB, which has much more variability.<ref name="becker">PMID: 15713683</ref> | + | <span style="color:red">The membrane composition of <i>Borrelia</i> is abundant in both OspA and OspB, and the two proteins share a 53% similarity in their primary sequences. Both are also expressed in the tick's gut and downregulated during feeding and aid in its survivability; however, OspA is overall less varied and reactive than OspB, which has much more variability.<ref name="becker">PMID: 15713683</ref> The relatively conserved sequence of OspA thus better lends itself to study than that of OspB and applications toward the development of a vaccine for Lyme disease for a broader range of strains. The first vaccine developed used a purified recombinant form of OspA and functioned in blocking transmission of the spirochetes from tick to host during feeding, killing them while still attached in the tick's gut.<ref name="connolly">PMID: 15864264</ref><ref name="battisti">PMID: 18779341</ref> |
| - | + | ||
</span> | </span> | ||
| - | + | ||
| + | The first vaccine against B. burgorferi sensu stricto was created targeting the OspA strain successfully 2 However this vaccine is unique in that it only works while Borrelia are still in the gut expressing OspA. (Ding) LYMErix, was discontinued in 2002 due to various weaknesses of the vaccine including its <80% efficacy, requirement for 3 doses, lack of data concerning the effects of the vaccine on children, and limitation of protection to the North American species of Borrelia. 6 To address this problem of international protection it would be helpful to create a chimera, mixing the OspA of different species. In order to do this the epitope of OspA should be studied. LA-2 <<SHOW FAB HIGHLIGHTED ON 1FJ1>> is a murine monoclonal antibody that binds strongly <<SHOW INTERACTION HIGHLIGHTED (ZOOMED IN) ON 1FJ1>> to OspA <<SHOW OSPA HIGHLIGHTED ON 1FJ1>>, and how effective a vaccine is correlated with LA-2 binding. 5 | ||
</p> | </p> | ||
| Line 107: | Line 107: | ||
http://www.nature.com/nrmicro/journal/v3/n5/abs/nrmicro1149.html<br> | http://www.nature.com/nrmicro/journal/v3/n5/abs/nrmicro1149.html<br> | ||
PMID: 15864264 | PMID: 15864264 | ||
| + | <ref name="connolly">PMID: 15864264</ref> | ||
<li>2 Rupprecht T, Koedel U, Fingerle V and Pfister H-W. 2008. The Pathogenesis of Lyme Neuroborreliosis: From Infection to Inflammation. Molecular Medicine 14(3-4): 205-212.<br> | <li>2 Rupprecht T, Koedel U, Fingerle V and Pfister H-W. 2008. The Pathogenesis of Lyme Neuroborreliosis: From Infection to Inflammation. Molecular Medicine 14(3-4): 205-212.<br> | ||
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2148032/<br> | http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2148032/<br> | ||
PMID: 18097481 | PMID: 18097481 | ||
| + | <ref name="rupprecht">PMID: 18097481</ref> | ||
<li>3 (now 4) Nigrovic L, Thompson K. 2007. Epidemiology and Infection. The Lyme Vaccine: A Cautionary Tale. 135(1)1-8.<br> | <li>3 (now 4) Nigrovic L, Thompson K. 2007. Epidemiology and Infection. The Lyme Vaccine: A Cautionary Tale. 135(1)1-8.<br> | ||
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2870557/<br> | http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2870557/<br> | ||
PMID: 16893489 | PMID: 16893489 | ||
| + | <ref name="nigrovic">PMID: 16893489</ref> | ||
<li>4 Battisti JM, Bono JL, Rosa PA, et al. 2008. Outer Surface Protein A Protects Lyme Disease Spirochetes from Acquired Host Immunity in the Tick Vector. Infect. Immun. 76(11): 5228-5237.<br> | <li>4 Battisti JM, Bono JL, Rosa PA, et al. 2008. Outer Surface Protein A Protects Lyme Disease Spirochetes from Acquired Host Immunity in the Tick Vector. Infect. Immun. 76(11): 5228-5237.<br> | ||
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2573341/<br> | http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2573341/<br> | ||
PMID: 18779341 | PMID: 18779341 | ||
| + | <ref name="battisti">PMID: 18779341</ref> | ||
<li>5 (now 3) Ding W, Huang X, Yang X, Dunn J, et al. 2000. Structural Identification of a Key Protective B-Cell Epitope in Lyme Disease Antigen Osp A, Journal of Molecular Biology 302(5): 1153-1164.<br> | <li>5 (now 3) Ding W, Huang X, Yang X, Dunn J, et al. 2000. Structural Identification of a Key Protective B-Cell Epitope in Lyme Disease Antigen Osp A, Journal of Molecular Biology 302(5): 1153-1164.<br> | ||
http://www.sciencedirect.com/science/article/pii/S0022283600941196<br> | http://www.sciencedirect.com/science/article/pii/S0022283600941196<br> | ||
PMID: 11183781 | PMID: 11183781 | ||
| + | <ref name="ding">PMID: 11183781</ref> | ||
<li>6 Plotkin S. 2011. Clinical Infectious Diseases. Correcting a Public Health Fiasco: The Need for a New Vaccine Against Lyme Disease. 52(3):s721-275.<br> | <li>6 Plotkin S. 2011. Clinical Infectious Diseases. Correcting a Public Health Fiasco: The Need for a New Vaccine Against Lyme Disease. 52(3):s721-275.<br> | ||
Revision as of 10:06, 2 May 2012
|
Outer Surface Protein A (OspA) is a major lipoprotein found on the surface of spirochetes from the genus Borrelia and is comprised of 21 anti-parallel β-strands and a single α-helix. OspA's expression is regulated at different points in time, from being downregulated during the tick's feeding process on its host to being upregulated in the host's cerebrospinal fluid (CSF) to induce inflammatory response, resulting in acute Lyme neuroborreliosis. OspA has also been used as a vector in working towards the development of a vaccine for Lyme disease.
NOTE: Red text indicates changes/additions I've made and this style is pertinent thoughts/questions we should consider/address. All of this will be cleaned up for the final submitted page. ~Kim
Contents |
Introduction
Lyme disease is caused by the spirochete Borrelia and spread via hard-bodied ticks belonging to the family Ixodidae. The Borrelia spirochetes are motile, helical organisms have several lipoproteins exposed on the surfaces of their membranes that invoke a response from host immune systems. The predominant group of these is classified as the outer surface proteins (Osps). Both the pathogenesis of Lyme disease as well as the host's immune response stem from the effects of the spirochete's presence and involvement in the system.[1] The major strain of Borrelia in the United States is Borrelia burgdorferi sensu stricto (Bb.), with 20-100 cases of Lyme disease being reported per 100,000 people. In Europe, Lyme disease cases are more prevalent, with 100-130 diagnoses per 100,000 people, but are caused by the spirochetes Borrelia afzelii (Ba.) and Borrelia garinii (Bg.) in addition to B. burgdorferi.[2][3] Do we want to mention the number of Borrelia strains but the fact that there are only 4 which are infectious to humans? - need a reference though
Lyme disease is a debilitating condition that begins with a characteristic bullseye rash known as an erythema migrans (EM) as well as the development of lesions on other parts of the body, not just at the site of the tick's attachment to its host. Secondary stage symptoms include affecting the heart, joints, and both the central and peripheral nervous systems. Borrelia have two major outer surface lipoproteins that are involved in Lyme disease: OspA and OspB. OspA is used in adhering to the tick's gut by binding with the tick receptor (TROSPA) Think a blurb about this would be useful- there isn't a wiki page for easy reference. During feeding, OspA is downregulated in order to evade an immune response from the incoming host blood into the gut, releasing the Borrelia from the gut wall and migrating into the tick's salivary glands, thereby allowing it to enter the host through the bite. This is evidenced by the fact that patients with Lyme disease have been found to not possess OspA antibodies in the early stages of the disease.[1][2] OspA is the protein most related to acute Lyme neuroborreliosis (LNB), the neurological manifestations of Lyme disease. We mention "two" proteins involved in Lyme Disease and just 'name' OspB but we don't even have a sentence or two about it in this part?
Once inside the host, the Borrelia has a great number of mechanisms available to actively suppress the host's immune system response and neutralize its effector mechanisms, such as the expression of another outer surface protein, OspC, which prevents susceptibility to the host's innate immunity and complement systems. Additionally, Borrelia is capable of suppressing many of its surface proteins to reduce its detectability, but can also utilize protective means by temporarily expressing them when needed.
OspA & Lyme Disease
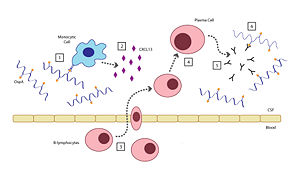
Acute Lyme Neuroborreliosis (LNB) is part of the second stage of Lyme disease in which the spirochete invades the peripheral and central nervous systems (CNS). Symptoms of LNB include: Bannwarth’s Syndrome, Lymphocytic Meningitis, and Cranial and Peripheral Neuritis. The presence of OspA in the cerebrospinal fluid (CSF) is responsible for this complex inflammatory response in the brain that leads to the neuroborreliosis.
There are six steps involved in the host's inflammatory response to OspA: [2]
- When the Borrelia enter the host’s CNS they encounter several different types of immune cells such as monocytes, macrophages, and dendritic cells. While in the CSF, outer surface protein A (OspA) is upregulated and it’s increased expression promotes recognition by a specific receptor on a monocyte.
- The OspA-bound monocyte then releases proinflammatory cytokines (i.e. interferon), as well as chemokines, such as CXCL13. In patients with LNB, there is an observed increase in the levels of these cytokines and chemokines in their CSF. The production of chemokines leads to the recruitment of other immune cells to the site of infection.
- B-lymphocytes respond to the new concentration gradient of CXCL13 between the blood and CSF and migrate into the CSF.
- B-lymphocytes undergo receptor-mediated endocytosis, consuming the OspA antigens present in the CSF, thereby triggering its activation. The B-lymphocytes then are able to differentiate and mature into plasma cells.
- The plasma cells create large quantities of anti-OspA antibodies specific to this strain of Borrelia and release them into the CSF.
- The anti-OspA antibodies will then bind to the OspA on the spirochete’s membrane, thus killing the Borrelia.
This process is two-sided in the sense that the OspA aids in the pathogenesis of new symptoms (neuroborreliosis) through the chemokine’s actions, as well as initiating the signaling cascade to destroy itself.
OspA Vaccination
Risk of developing Lyme disease can be mitigated by staying clear of areas with populations of ticks, wearing proper attire to minimize easily bitten areas of the body, and using insect repellents containing DEET (N,N-diethy-m-toluamide). However, another effective means for prevention could be possible by using an outer surface protein from Borrelia in the development of a vaccine.[4]
The membrane composition of Borrelia is abundant in both OspA and OspB, and the two proteins share a 53% similarity in their primary sequences. Both are also expressed in the tick's gut and downregulated during feeding and aid in its survivability; however, OspA is overall less varied and reactive than OspB, which has much more variability.[5] The relatively conserved sequence of OspA thus better lends itself to study than that of OspB and applications toward the development of a vaccine for Lyme disease for a broader range of strains. The first vaccine developed used a purified recombinant form of OspA and functioned in blocking transmission of the spirochetes from tick to host during feeding, killing them while still attached in the tick's gut.[1][6] The first vaccine against B. burgorferi sensu stricto was created targeting the OspA strain successfully 2 However this vaccine is unique in that it only works while Borrelia are still in the gut expressing OspA. (Ding) LYMErix, was discontinued in 2002 due to various weaknesses of the vaccine including its <80% efficacy, requirement for 3 doses, lack of data concerning the effects of the vaccine on children, and limitation of protection to the North American species of Borrelia. 6 To address this problem of international protection it would be helpful to create a chimera, mixing the OspA of different species. In order to do this the epitope of OspA should be studied. LA-2 <<SHOW FAB HIGHLIGHTED ON 1FJ1>> is a murine monoclonal antibody that binds strongly <<SHOW INTERACTION HIGHLIGHTED (ZOOMED IN) ON 1FJ1>> to OspA <<SHOW OSPA HIGHLIGHTED ON 1FJ1>>, and how effective a vaccine is correlated with LA-2 binding. 5
Structure of OspA
|
OspA is made of 21 anti-parallel β-strands and a single α-helix. 5 OspA is unique due to its dumbbell shape that contains two globular domains connected by a single layer β-sheet. 7 There are located at the C-terminus that are important in binding, and .
Loop 1 <<LOOP 1>>, residues 206 and 216, has an important role in binding due to a large exposed surface area, high mobility. Loop 2 <<LOOP 2>>, residues 224-233, and Loop 3 <<LOOP 3>>, residues 246-257, are also areas that are involved in binding. LA-2 recognizes OspA Bb, but does not recognize OspA from Bg.and Ba. Between Bb. and Ba. genetic sequences are generally invariant, but two residues change between the species, ALA 208 <<ALA 208>> in Bb. is GLN in Ba., and ASN 251 <<ASN 251>> in Bb. is ALA in Ba.. Bg. has more variation and in addition to the previous two differences, has at least one more difference, where ALA 215 <<ALA 215>> in Bb. is LYS, Bg. sometimes also has a deletion at Bb.’s ALA 208. LA-2 and OspA of Bb. form a tight interface when binding, and the longer GLN sidechain found in Ba. and Bg. is more difficult to accommodate, causing less binding. A chimera that was weakly recognized by LA-2 was made with parts of loop 1 from Bb., and loops 2 and 3 from Bg. 5 Recently, a different kind of chimera has been made which combined the proximal region of Bb. and distal region of Ba., and was able to successfully protect mice from both species. 8
Reference List of Available Scenes for OspA
- (close up)
- (Ala208, Ala215 and Asn251 in B. burgdorferi, also hides R-groups)
- of Ala208, Ala215 and Asn251
In-Prog References
Not fully structured yet with -ref- tags, just organizing all reference information for ease
- 1 Connolly, SE and Benach JL. 2005. The Versatile Roles of Antibodies in Borrelia Infections. Microbiology 3: 411-420.
http://www.nature.com/nrmicro/journal/v3/n5/abs/nrmicro1149.html
PMID: 15864264 [1] - 2 Rupprecht T, Koedel U, Fingerle V and Pfister H-W. 2008. The Pathogenesis of Lyme Neuroborreliosis: From Infection to Inflammation. Molecular Medicine 14(3-4): 205-212.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2148032/
PMID: 18097481 [2] - 3 (now 4) Nigrovic L, Thompson K. 2007. Epidemiology and Infection. The Lyme Vaccine: A Cautionary Tale. 135(1)1-8.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2870557/
PMID: 16893489 [4] - 4 Battisti JM, Bono JL, Rosa PA, et al. 2008. Outer Surface Protein A Protects Lyme Disease Spirochetes from Acquired Host Immunity in the Tick Vector. Infect. Immun. 76(11): 5228-5237.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2573341/
PMID: 18779341 [6] - 5 (now 3) Ding W, Huang X, Yang X, Dunn J, et al. 2000. Structural Identification of a Key Protective B-Cell Epitope in Lyme Disease Antigen Osp A, Journal of Molecular Biology 302(5): 1153-1164.
http://www.sciencedirect.com/science/article/pii/S0022283600941196
PMID: 11183781 [3] - 6 Plotkin S. 2011. Clinical Infectious Diseases. Correcting a Public Health Fiasco: The Need for a New Vaccine Against Lyme Disease. 52(3):s721-275.
- 7 Koide? Who used this?
- 8 Livey I, O’Rourke M, Traweger A, Savidis-Dacho H, Crowe B, Barrett P, Yang X, Dunn J, Luft B. 2011. Clinical Infectious Diseases. A new approach to a Lyme Disease Vaccine. 52(3):s266-s270.
- 9 (now 5) Becker M et al. 2005. Structural Investigation of Borrelia burgdorferi OspB, a BactericidalFab Target. JOURNAL OF BIOLOGICAL CHEMISTRY. 280(17):17363–17370.
http://www.jbc.org/content/280/17/17363.long
PMID: 15713683
References
- ↑ 1.0 1.1 1.2 1.3 Connolly SE, Benach JL. The versatile roles of antibodies in Borrelia infections. Nat Rev Microbiol. 2005 May;3(5):411-20. PMID:15864264 doi:10.1038/nrmicro1149
- ↑ 2.0 2.1 2.2 2.3 Rupprecht TA, Koedel U, Fingerle V, Pfister HW. The pathogenesis of lyme neuroborreliosis: from infection to inflammation. Mol Med. 2008 Mar-Apr;14(3-4):205-12. PMID:18097481 doi:10.2119/2007-00091.Rupprecht
- ↑ 3.0 3.1 Ding W, Huang X, Yang X, Dunn JJ, Luft BJ, Koide S, Lawson CL. Structural identification of a key protective B-cell epitope in Lyme disease antigen OspA. J Mol Biol. 2000 Oct 6;302(5):1153-64. PMID:11183781 doi:10.1006/jmbi.2000.4119
- ↑ 4.0 4.1 Nigrovic LE, Thompson KM. The Lyme vaccine: a cautionary tale. Epidemiol Infect. 2007 Jan;135(1):1-8. Epub 2006 Aug 8. PMID:16893489 doi:10.1017/S0950268806007096
- ↑ Becker M, Bunikis J, Lade BD, Dunn JJ, Barbour AG, Lawson CL. Structural investigation of Borrelia burgdorferi OspB, a bactericidal Fab target. J Biol Chem. 2005 Apr 29;280(17):17363-70. Epub 2005 Feb 15. PMID:15713683 doi:10.1074/jbc.M412842200
- ↑ 6.0 6.1 Battisti JM, Bono JL, Rosa PA, Schrumpf ME, Schwan TG, Policastro PF. Outer surface protein A protects Lyme disease spirochetes from acquired host immunity in the tick vector. Infect Immun. 2008 Nov;76(11):5228-37. Epub 2008 Sep 8. PMID:18779341 doi:10.1128/IAI.00410-08
External Links
- World Health Organization: Lyme Disease
- PubMed Health: Lyme Disease
- American Lyme Disease Foundation
Proteopedia Page Contributors and Editors
Kimberly Slade, Cara Lin, Andrea Mullen, Jenny Kim Kim