Sandbox Reserved 476
From Proteopedia
| Line 34: | Line 34: | ||
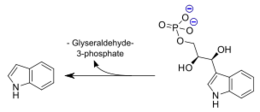
As for the mechanism, in the first of these two steps, it is believed that the αGlu49 and αAsp60 residues are directly involved in the [http://en.wikipedia.org/wiki/Aldol_reaction: retro-aldol cleavage] of indole-3-glycerol phosphate into the products indole and glyceraldehyde-3-phosphate. Within this step is a transition state in which the aldol molecule must isomerize, which limite the rate of this reaction. | As for the mechanism, in the first of these two steps, it is believed that the αGlu49 and αAsp60 residues are directly involved in the [http://en.wikipedia.org/wiki/Aldol_reaction: retro-aldol cleavage] of indole-3-glycerol phosphate into the products indole and glyceraldehyde-3-phosphate. Within this step is a transition state in which the aldol molecule must isomerize, which limite the rate of this reaction. | ||
| - | [[Image:trpsynthstep2.PNG |left|260px|alt=text|β-catalyzed reaction]] | + | [[Image:trpsynthstep2.PNG |left|260px|alt=text|frame=true|β-catalyzed reaction]] |
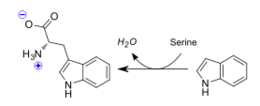
| - | The β-catalyzed reaction, and final step in biosynthesis of the amino acid, is that of the PLP co-factor dependent replacement of the hydroxyl group of serine's side chain with the indole group produced by the previous step. All current data points to the involvement of the βLys87, βGlu109, and βSer377 residues in the catalysis of this reaction, though the specific mechanism by which this is performed remains undetermined. | ||
| + | |||
| + | |||
| + | |||
| + | The β-catalyzed reaction, and final step in biosynthesis of the amino acid, is that of the PLP co-factor dependent replacement of the hydroxyl group of serine's side chain with the indole group produced by the previous step. All current data points to the involvement of the βLys87, βGlu109, and βSer377 residues in the catalysis of this reaction, though the specific mechanism by which this is performed remains undetermined. | ||
| Line 44: | Line 47: | ||
| - | =='''Applications'''== | ||
Revision as of 19:00, 2 May 2012
| This Sandbox is Reserved from 13/03/2012, through 01/06/2012 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 451 through Sandbox Reserved 500. | |||||||
To get started:
More help: Help:Editing For more help, look at this link: http://www.proteopedia.org/wiki/index.php/Help:Getting_Started_in_Proteopedia
Tryptophan Synthase
Tryptophan synthase is an enzyme present in many organism families, with the notable exception of the kingdom Animalia. The role of this enzyme is to catalyze the final two steps of tryptophan biosynthesis; the first of these being the reversible conversion of indole-3-glycerol phosphate to indole(trp precursor) and glyceraldehyde-3-phosphate(known substrate "G3P" within Glycolysis pathway), and the latter being the condensation reaction of indole with serine to form the ultimate products of L-tryptophan and water. It is also of significance that Tryptophan synthase was one of the first enzymes possesing two catalytic activities of great interest in the scientific community, as well as the first enzyme discovered to utilize the process of substrate channeling.[1]
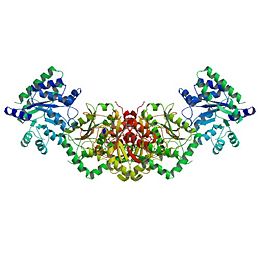
StructureTryptophan synthase is composed of α(27kDa in mass) and β(43 kDa in mass) subunits, most commonly arranged as an α-ββ-α complex(see left). Primary structural features include a TIM barrel conformation of the α subunit while it's counterpart is observed with a type II conformation. With the complexing of the subunits comes major structural changes, resulting in the enzyme containing interaction of the domain of the β subunit with the α-loop2 of the α-subunit as well as the αGly181 with βSer178 residues.
FunctionThe function of this enzyme is that of catalyzing the final two steps of the tryptophan biosynthesis pathway, and as such is required for producing the amino acid. Because this enzyme is not present in animals, it has been classified as one of the eight essential amino acids required in the human diet.
References
|