This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 480
From Proteopedia
| Line 5: | Line 5: | ||
<Structure load='2pah' size='400' frame='true' align='right' caption='Phenylalanine Hydroxylase' scene='Insert optional scene name here'/> | <Structure load='2pah' size='400' frame='true' align='right' caption='Phenylalanine Hydroxylase' scene='Insert optional scene name here'/> | ||
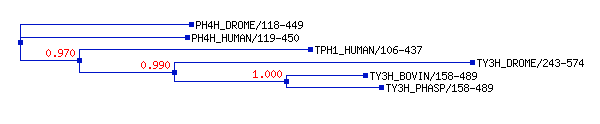
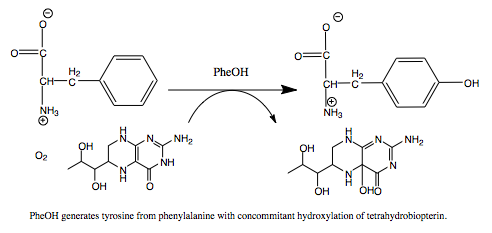
| - | Phenylalanine Hydroxylase (PheOH) is a type of enzyme involved in the catalization of [http://en.wikipedia.org/wiki/Phenylalanine phenylalanine] into [http://en.wikipedia.org/wiki/Tyrosine tyrosine].It belongs to the family of tetrahydrobiopterin (BH4) dependent aromatic amino acid hydrolase family of proteins.<ref name="BH4_H">PMID: 14640675< | + | Phenylalanine Hydroxylase (PheOH) is a type of enzyme involved in the catalization of [http://en.wikipedia.org/wiki/Phenylalanine phenylalanine] into [http://en.wikipedia.org/wiki/Tyrosine tyrosine].It belongs to the family of tetrahydrobiopterin (BH4) dependent aromatic amino acid hydrolase family of proteins.<ref name="BH4_H">PMID: 14640675</ref> Some other enzymes in this family include tyrosine hydroxylase and tryptophan hydroxylase. Below is a small scale phylogenetic tree of the different enzymes in this family. The human phenylalanine hydroxylase is labeled here as PH4H_HUMAN/119-450. |
[[Image:Phylogenetic_tree_BH4_H.gif]] | [[Image:Phylogenetic_tree_BH4_H.gif]] | ||
These enzymes add a hydroxyl group to the side chain of their corresponding amino acid. In order for this reaction to occur they are all dependent on BH4, as it acts as an electron carrier for the reaction. BH4 dependent aromatic amino acid hydroxylases are found in eukaryotic and some bacteria cells. In mammals PheOH is found mostly in the liver, where it can breakdown excess Phe from the diet. Mutations in this enzyme can lead to phenylketonuria (PKU), where phenylalanine builds up in the body and can cause mental retardation. Hyperphenylalaninemia is a similar disorder, which also involves a disruption in the metabolic pathway of Phe. | These enzymes add a hydroxyl group to the side chain of their corresponding amino acid. In order for this reaction to occur they are all dependent on BH4, as it acts as an electron carrier for the reaction. BH4 dependent aromatic amino acid hydroxylases are found in eukaryotic and some bacteria cells. In mammals PheOH is found mostly in the liver, where it can breakdown excess Phe from the diet. Mutations in this enzyme can lead to phenylketonuria (PKU), where phenylalanine builds up in the body and can cause mental retardation. Hyperphenylalaninemia is a similar disorder, which also involves a disruption in the metabolic pathway of Phe. | ||
| Line 11: | Line 11: | ||
== Structure == | == Structure == | ||
[[Image:Tetrameric_2pah_mm1.png | thumb | Tetrameric Structure of PheOH]] | [[Image:Tetrameric_2pah_mm1.png | thumb | Tetrameric Structure of PheOH]] | ||
| - | The regulatory domain affects the function of the active site. When the regulatory domain is <scene name='Sandbox_Reserved_480/Phosphorylated_pheoh/2'>phosphorylated</scene>, the active site is closed off and prevented form interacting with the substrates. In this scene the chain is colored from N-terminus to C-terminus blue to red and the active site and Fe (III) is colored black. In the <scene name='Sandbox_Reserved_480/Dephosphorylated_pheoh/1'>dephosphorylated</scene> PheOH, the active site is open to the subtrates and can convert them to products. | + | These hydroxylases are tetramers which contain homologous subunits. Each subunit is made up of three domains: the regulatory domain, catalytic domain, and tetramerization domain. The regulatory domain is in the N-terminus region of the chain. The catalytic domain is found in the middle nearing the C-terminus and the last set of residues make up the tetramerization domain, which helps form the tetrameric structure. The regulatory domain affects the function of the active site. When the regulatory domain is <scene name='Sandbox_Reserved_480/Phosphorylated_pheoh/2'>phosphorylated</scene>, the active site is closed off and prevented form interacting with the substrates. In this scene the chain is colored from N-terminus to C-terminus blue to red and the active site and Fe (III) is colored black. In the <scene name='Sandbox_Reserved_480/Dephosphorylated_pheoh/1'>dephosphorylated</scene> PheOH, the active site is open to the subtrates and can convert them to products. |
| - | PheOH is a tetrameric enzyme, consisting of 2 asymmetric components. | + | PheOH is a tetrameric enzyme, consisting of 2 asymmetric components. In this scene it can be viewed as a <scene name='Sandbox_Reserved_480/Dimer/1'>dimer</scene>. This is the <scene name='Sandbox_Reserved_480/Active_site/3'>active</scene> site within the subunit. |
This enzyme has two <scene name='Sandbox_Reserved_480/Ligands/1'>ligands</scene>, Fe (III) and . | This enzyme has two <scene name='Sandbox_Reserved_480/Ligands/1'>ligands</scene>, Fe (III) and . | ||
== Phenylketonuria == | == Phenylketonuria == | ||
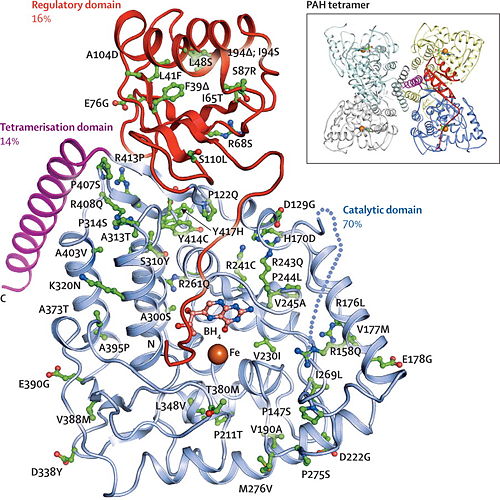
| - | + | The image below depicts the possible mutations of the PheOH subunit in each of the three domains. It also gives a representative picture of the tetrameric structure.<ref name="PKU">Nenad Blau, Francjan J van Spronsen, Harvey L Levy. ''Phenylketonuria.'' The Lancet. 376(9750): 1417-1427 (2010)[http://www.sciencedirect.com/science/article/pii/S0140673610609610 DOI: 10.1016/S0140-6736(10)60961-0]</ref> | |
[[Image:Domain phenylketonuria 4.jpg | 500px]] | [[Image:Domain phenylketonuria 4.jpg | 500px]] | ||
== Mechanism == | == Mechanism == | ||
Revision as of 01:24, 3 May 2012
| This Sandbox is Reserved from 13/03/2012, through 01/06/2012 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 451 through Sandbox Reserved 500. | |||||||
To get started:
More help: Help:Editing For more help, look at this link: http://www.proteopedia.org/wiki/index.php/Help:Getting_Started_in_Proteopedia
Phenylalanine Hydroxylase
Phenylalanine Hydroxylase (PheOH) is a type of enzyme involved in the catalization of phenylalanine into tyrosine.It belongs to the family of tetrahydrobiopterin (BH4) dependent aromatic amino acid hydrolase family of proteins.[1] Some other enzymes in this family include tyrosine hydroxylase and tryptophan hydroxylase. Below is a small scale phylogenetic tree of the different enzymes in this family. The human phenylalanine hydroxylase is labeled here as PH4H_HUMAN/119-450. These enzymes add a hydroxyl group to the side chain of their corresponding amino acid. In order for this reaction to occur they are all dependent on BH4, as it acts as an electron carrier for the reaction. BH4 dependent aromatic amino acid hydroxylases are found in eukaryotic and some bacteria cells. In mammals PheOH is found mostly in the liver, where it can breakdown excess Phe from the diet. Mutations in this enzyme can lead to phenylketonuria (PKU), where phenylalanine builds up in the body and can cause mental retardation. Hyperphenylalaninemia is a similar disorder, which also involves a disruption in the metabolic pathway of Phe. StructureThese hydroxylases are tetramers which contain homologous subunits. Each subunit is made up of three domains: the regulatory domain, catalytic domain, and tetramerization domain. The regulatory domain is in the N-terminus region of the chain. The catalytic domain is found in the middle nearing the C-terminus and the last set of residues make up the tetramerization domain, which helps form the tetrameric structure. The regulatory domain affects the function of the active site. When the regulatory domain is , the active site is closed off and prevented form interacting with the substrates. In this scene the chain is colored from N-terminus to C-terminus blue to red and the active site and Fe (III) is colored black. In the PheOH, the active site is open to the subtrates and can convert them to products. PheOH is a tetrameric enzyme, consisting of 2 asymmetric components. In this scene it can be viewed as a . This is the site within the subunit. This enzyme has two , Fe (III) and . PhenylketonuriaThe image below depicts the possible mutations of the PheOH subunit in each of the three domains. It also gives a representative picture of the tetrameric structure.[2]
MechanismReferences
|