To get started:
- Click the edit this page tab at the top. Save the page after each step, then edit it again.
- Click the 3D button (when editing, above the wikitext box) to insert Jmol.
- show the Scene authoring tools, create a molecular scene, and save it. Copy the green link into the page.
- Add a description of your scene. Use the buttons above the wikitext box for bold, italics, links, headlines, etc.
More help: Help:Editing
For more help, look at this link:
http://www.proteopedia.org/wiki/index.php/Help:Getting_Started_in_Proteopedia
Creatine Kinase
Creatine Kinase (CK), sometimes referred to as Creatine Phosphokinase (CPK), is an enzyme (EC 2.7.3.2). CK is classified as a transferase, which means it facilitates the transfer of a group from one molecule to another.
AX + B ---> A + BX
The first three numbers of its EC number indicate that it is a phosphotransferase with a nitrogenous group as the acceptor. CK is a very important enzyme for all organisms, as it catalyzes the conversion of creatine into phosphocreatine. Phosphocreatine is used as an energy source for high energy need cells such as smooth muscle cells. CK is clinically relevant in blood serum assays in that an elevated CK level might indicate muscle wasting or myocardial infarction.
Structure
There are four major isozymes in the CK family and have been characterized on the basis of differences in gene and amino acid sequence, as well as tissue localization and immunogenicity. The four isozymes are the muscle (MM-CK), brain (BB-CK), mitochondrial ubiquitous (Miu-CK) and mitochondrial sarcomeric (Mis-CK).
The functional entity of the two mitochondrial CK isozymes is an octamer consisting of four dimers each.
In the first 3D model you can see the creatine and ADP analog sites highlighted in pink.
Mechanism
Creatine Kinase catalyzes the reversible transfer of a phosporyl group from MgATP to creatine. This yields phosphocreatine (PCr) and MgADP as seen below.
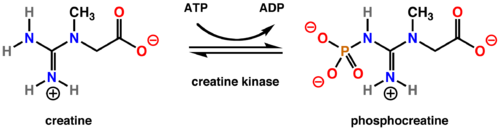
Real World Application
As mention previously, CK is routinely assayed in blood serum samples in a clinical setting. High levels of CK along with other factors such as lactate dehydrogenase and aspartate aminotransferase have become reliable indicators of myocardial infarctions. These levels are sometimes drawn in patients that have not experienced clear heart attack symptoms but are at risk for such occurences. Normal values are between 60 and 410 IU/L, but these values do not represent the specific isozyme present. Testing of CK levels are also performed in the case of renal discomfort or muscle wasting diseases. Elevated levels might indicate malnourishment, injury, myocarditis, hypothyroidism, or use of statin medications. Lower than average CK levels rheumatoid arthritis or certain hepatic diseases.
References
[1]
[2]
[3]
[4]
|

