We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 470
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
==Structure== | ==Structure== | ||
---- | ---- | ||
| - | *While GAPDH possesses four cysteine residues, its <scene name='Sandbox_Reserved_470/Active_site_gapdh/1'>active site</scene> is located around <scene name='Sandbox_Reserved_470/Cysteine_active_site/1'>Cysteine 149</scene>. | + | *While GAPDH possesses four cysteine residues, its <scene name='Sandbox_Reserved_470/Active_site_gapdh/1'>active site</scene> is located around <scene name='Sandbox_Reserved_470/Cysteine_active_site/1'>Cysteine 149</scene>, which is responsible for inhibition of glycolysis and fermentation by iodoacetic acid. '''<ref>''' Boyer, Paul D.. "The Enzymes - Paul D. Boyer - Google Books." Google Books. N.p., n.d. Web. 1 May 2012. '''[<http://books.google.com/books?id=r6SRFsxYFYwC&pg=PA20&lpg=PA20&dq=GAPDH+cysteine+149&source=bl&ots=0CWwdduzGB&sig=Lb8PtWq7EGdwyvdAnFI_rHYG3tU&hl=en&sa=X&ei=2PyhT-WtHJSy8QTjtsnTCA&ved=0CDMQ6AEwAg#v=onepage&q=GAPDH%20cysteine%20149&f=false>]'''. '''</ref>''' |
*GAPDH has two domains, a GAPDH-like, C-terminal domain, and an NAD Binding Domain. | *GAPDH has two domains, a GAPDH-like, C-terminal domain, and an NAD Binding Domain. | ||
:The <scene name='Sandbox_Reserved_470/Nad_binding_domain/3'>NAD (Nucleotide) Binding Domain </scene> is an Alpha Beta 3-Layer(α-β-α) Sandwich, including amino acids 1-138 and 301-340 on chains O and Q . Its [http://en.wikipedia.org/wiki/CATH CATH] reports a Rossmann fold and a classification as an [http://en.wikipedia.org/wiki/Oxidoreductase oxidoreductase]. | :The <scene name='Sandbox_Reserved_470/Nad_binding_domain/3'>NAD (Nucleotide) Binding Domain </scene> is an Alpha Beta 3-Layer(α-β-α) Sandwich, including amino acids 1-138 and 301-340 on chains O and Q . Its [http://en.wikipedia.org/wiki/CATH CATH] reports a Rossmann fold and a classification as an [http://en.wikipedia.org/wiki/Oxidoreductase oxidoreductase]. | ||
| Line 15: | Line 15: | ||
*Depending on the environment, including variations in temperature and acidity, GAPDH can have different structural qualities and physical properties. In some cases, it is observed as a twinned structure with an α/β barrel. | *Depending on the environment, including variations in temperature and acidity, GAPDH can have different structural qualities and physical properties. In some cases, it is observed as a twinned structure with an α/β barrel. | ||
| + | [[Image:Cysteine_active_site.jpeg]] | ||
==Importance== | ==Importance== | ||
---- | ---- | ||
| Line 43: | Line 44: | ||
==References== | ==References== | ||
<references/> | <references/> | ||
| + | #↑Boyer, Paul D.. "The Enzymes - Paul D. Boyer - Google Books." Google Books. N.p., n.d. Web. 1 May 2012. '''<ref>''' Boyer, Paul D.. "The Enzymes - Paul D. Boyer - Google Books." Google Books. N.p., n.d. Web. 1 May 2012. '''[<http://books.google.com/books?id=r6SRFsxYFYwC&pg=PA20&lpg=PA20&dq=GAPDH+cysteine+149&source=bl&ots=0CWwdduzGB&sig=Lb8PtWq7EGdwyvdAnFI_rHYG3tU&hl=en&sa=X&ei=2PyhT-WtHJSy8QTjtsnTCA&ved=0CDMQ6AEwAg#v=onepage&q=GAPDH%20cysteine%20149&f=false>]'''. '''</ref>''' | ||
#↑"GAPDH glyceraldehyde-3-phosphate dehydrogenase [Homo sapiens] - Gene - NCBI." National Center for Biotechnology Information. N.p., n.d. Web. 1 May 2012. '''<ref>'''"GAPDH glyceraldehyde-3-phosphate dehydrogenase [Homo sapiens] - Gene - NCBI." National Center for Biotechnology Information. N.p., n.d. Web. 1 May 2012. '''[<http://www.ncbi.nlm.nih.gov/gene/2597>]'''. '''<ref/>''' | #↑"GAPDH glyceraldehyde-3-phosphate dehydrogenase [Homo sapiens] - Gene - NCBI." National Center for Biotechnology Information. N.p., n.d. Web. 1 May 2012. '''<ref>'''"GAPDH glyceraldehyde-3-phosphate dehydrogenase [Homo sapiens] - Gene - NCBI." National Center for Biotechnology Information. N.p., n.d. Web. 1 May 2012. '''[<http://www.ncbi.nlm.nih.gov/gene/2597>]'''. '''<ref/>''' | ||
#↑"Glyceraldehyde 3-phosphate dehydrogenase." Stanford University. N.p., n.d. Web. 22 Apr. 2012. '''<ref>'''"Glyceraldehyde 3-phosphate dehydrogenase." Stanford University. N.p., n.d. Web. 22 Apr. 2012. '''[<http://www.stanford.edu/~mmogri/bio/gapdh/structure.html>]'''. '''</ref>''' | #↑"Glyceraldehyde 3-phosphate dehydrogenase." Stanford University. N.p., n.d. Web. 22 Apr. 2012. '''<ref>'''"Glyceraldehyde 3-phosphate dehydrogenase." Stanford University. N.p., n.d. Web. 22 Apr. 2012. '''[<http://www.stanford.edu/~mmogri/bio/gapdh/structure.html>]'''. '''</ref>''' | ||
Revision as of 03:46, 3 May 2012
| This Sandbox is Reserved from 13/03/2012, through 01/06/2012 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 451 through Sandbox Reserved 500. | |||||||
To get started:
More help: Help:Editing For more help, look at this link: http://www.proteopedia.org/wiki/index.php/Help:Getting_Started_in_Proteopedia
Introduction
Structure
Image:Cysteine active site.jpeg ImportanceRole in Glycolysis:The Steps:
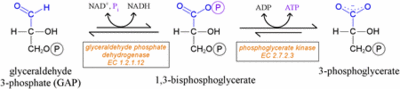
GAPDH catalyzes the conversion of glyceraldyhyde-3-phosphate at carbon 1 to 1,3-bisphosphoglycerate (1,3-BPG). The Reactions:
The Mechanism:
Other roles:
GAPDH was also found to be involved in ER to Golgi transport because it is recruited by rab2 to vesicular-tubular clusters of the endoplasmic reticulum where it helps form COP 1 vesicles.
Use in the lab:Quantitation:
Qualification and Analysis:
References
|