Nitrite reductase
From Proteopedia
| Line 1: | Line 1: | ||
{{STRUCTURE_1as8| PDB=1as8 | SIZE=500| SCENE= |right|CAPTION=Copper-containing nitric reductase complex with NO2 [[1as8]] }} | {{STRUCTURE_1as8| PDB=1as8 | SIZE=500| SCENE= |right|CAPTION=Copper-containing nitric reductase complex with NO2 [[1as8]] }} | ||
| - | '''Nitrite reductase''' (NIR) catalyzes the reduction of NO2 to NO. There are 2 classes of NIR: (1) A heme-containing cytochrome Cd type NIR. This enzyme contains 4 heme groups. Its d-type heme group binds | + | '''Nitrite reductase''' (NIR) catalyzes the reduction of NO2 to NO. There are 2 classes of NIR: (1) A heme-containing cytochrome Cd type NIR. This enzyme contains 4 heme groups. Its d-type heme group binds NO<sub>2</sub>. (2) A copper-containing NIR which produces NO<sub>2</sub>. Under anaerobic conditions bacteria rely on the reduction of nitrogen oxide species to obtain energy. NIR is part of the nitrogen cycle used fot this purpose. |
| - | Cytochrome c nitrite reductase (ccNIR | + | Cytochrome c nitrite reductase (ccNIR) is a central enzyme of the nitrogen cycle. It binds nitrite, and reduces it by transferring 6 electrons to form ammonia. This ammonia can then be utilized to synthesize nitrogen containing molecules such as amino acids or nucleic acids. However, ccNiR’s primary role is to help extract energy from the reduction; ammonia is simply a potentially useful byproduct. In general, heterotrophic organisms feed on electron-rich substances such as sugars or fatty acids. During the metabolism of these substances large numbers of electrons are produced. Many organisms use oxygen as the final acceptor of these electrons, in which case water is formed. However, some organisms can use alternative electron acceptors such as nitrite, which is where ccNiR comes in. |
<StructureSection load='Cca.pdb' size='500' side='right' scene='Journal:JBIC:16/Cv/2' caption=''> | <StructureSection load='Cca.pdb' size='500' side='right' scene='Journal:JBIC:16/Cv/2' caption=''> | ||
| - | '''Laue Crystal Structure of ''Shewanella oneidensis'' Cytochrome c Nitrite Reductase from a High-yield Expression System''' | + | '''Laue Crystal Structure of ''Shewanella oneidensis'' Cytochrome c Nitrite Reductase from a High-yield Expression System''' <ref name="Youngblut">doi 10.1007/s00775-012-0885-0</ref> |
| - | <ref name="Youngblut">doi 10.1007/s00775-012-0885-0</ref> | + | |
The ccNiR described here is produced by the ''Shewanella oneidensis'' bacterium, which is remarkable in its own right due to the large number of electron acceptors that it can utilize. ''Shewanella'' is a facultative anaerobe, which means that it will use oxygen if available, but in the absence of oxygen can get rid of its electrons by dumping them on a wide range of alternate acceptors, of which nitrite is only one example. To handle the electron flow ''Shewanella'' uses a large number of promiscuous <scene name='Journal:JBIC:16/Cv/8'>c-heme</scene> containing electron transfer proteins. Indeed, ''Shewanella'' is exceptionally adept at producing c-heme proteins under fast-growth conditions, which many bacteria commonly used for large-scale laboratory gene expression, such as ''E. coli'', are incapable of unless they are first extensively reprogrammed genetically. Since ''Shewanella'' can be easily grown in the lab, and can naturally and easily produce c-hemes, it is an ideal host for generating large quantities of c-heme proteins such as ccNiR. | The ccNiR described here is produced by the ''Shewanella oneidensis'' bacterium, which is remarkable in its own right due to the large number of electron acceptors that it can utilize. ''Shewanella'' is a facultative anaerobe, which means that it will use oxygen if available, but in the absence of oxygen can get rid of its electrons by dumping them on a wide range of alternate acceptors, of which nitrite is only one example. To handle the electron flow ''Shewanella'' uses a large number of promiscuous <scene name='Journal:JBIC:16/Cv/8'>c-heme</scene> containing electron transfer proteins. Indeed, ''Shewanella'' is exceptionally adept at producing c-heme proteins under fast-growth conditions, which many bacteria commonly used for large-scale laboratory gene expression, such as ''E. coli'', are incapable of unless they are first extensively reprogrammed genetically. Since ''Shewanella'' can be easily grown in the lab, and can naturally and easily produce c-hemes, it is an ideal host for generating large quantities of c-heme proteins such as ccNiR. | ||
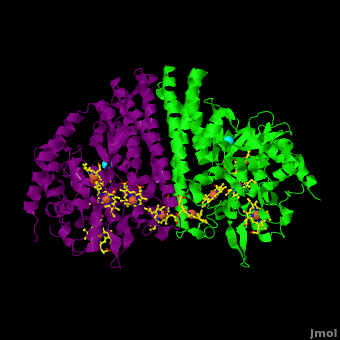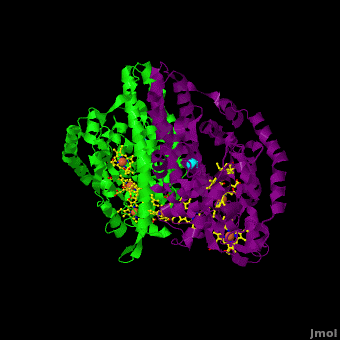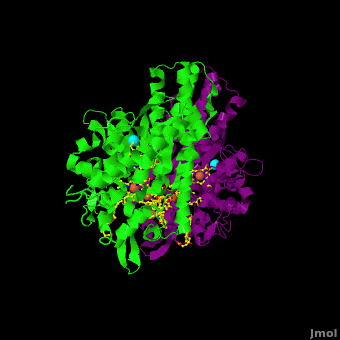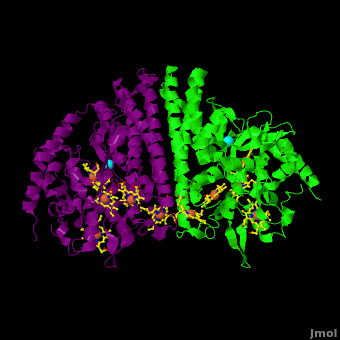
The 2012 paper by Youngblut et al. <ref name="Youngblut">none yet</ref> describes a genetically modified ''Shewanella'' strain that can produce 20 – 40 times more ccNiR per liter of culture than the wild type bacterium. The ccNir so produced can be purified easily and in large amounts. This result is important because c-heme proteins have historically proved difficult to over-express in traditional vectors such as ''E. coli''. With large quantities of ''Shewanella'' ccNIR available, Youngblut et al <ref name="Youngblut">none yet</ref> were able to obtain the crystal structure ([[3ubr]]) and do a variety of experiments. The ccNIR consists of <scene name='Journal:JBIC:16/Cv/4'>two equal subunits</scene> (<font color='darkmagenta'><b>colored in darkmagenta</b></font> and <span style="color:lime;background-color:black;font-weight:bold;">in green</span>) with <scene name='Journal:JBIC:16/Cv/5'>five c-hemes each</scene>. In the oxidized ccNIR all central heme irons are Fe3+. They can be subsequently reduced to Fe2+ either by reducing agents or electrochemically. An important conclusion of the paper is that electrons added to ccNiR are likely <scene name='Journal:JBIC:16/Cv/6'>delocalized over several hemes</scene>, rather than localized on individual hemes. | The 2012 paper by Youngblut et al. <ref name="Youngblut">none yet</ref> describes a genetically modified ''Shewanella'' strain that can produce 20 – 40 times more ccNiR per liter of culture than the wild type bacterium. The ccNir so produced can be purified easily and in large amounts. This result is important because c-heme proteins have historically proved difficult to over-express in traditional vectors such as ''E. coli''. With large quantities of ''Shewanella'' ccNIR available, Youngblut et al <ref name="Youngblut">none yet</ref> were able to obtain the crystal structure ([[3ubr]]) and do a variety of experiments. The ccNIR consists of <scene name='Journal:JBIC:16/Cv/4'>two equal subunits</scene> (<font color='darkmagenta'><b>colored in darkmagenta</b></font> and <span style="color:lime;background-color:black;font-weight:bold;">in green</span>) with <scene name='Journal:JBIC:16/Cv/5'>five c-hemes each</scene>. In the oxidized ccNIR all central heme irons are Fe3+. They can be subsequently reduced to Fe2+ either by reducing agents or electrochemically. An important conclusion of the paper is that electrons added to ccNiR are likely <scene name='Journal:JBIC:16/Cv/6'>delocalized over several hemes</scene>, rather than localized on individual hemes. | ||
Revision as of 10:24, 14 May 2012
Nitrite reductase (NIR) catalyzes the reduction of NO2 to NO. There are 2 classes of NIR: (1) A heme-containing cytochrome Cd type NIR. This enzyme contains 4 heme groups. Its d-type heme group binds NO2. (2) A copper-containing NIR which produces NO2. Under anaerobic conditions bacteria rely on the reduction of nitrogen oxide species to obtain energy. NIR is part of the nitrogen cycle used fot this purpose.
Cytochrome c nitrite reductase (ccNIR) is a central enzyme of the nitrogen cycle. It binds nitrite, and reduces it by transferring 6 electrons to form ammonia. This ammonia can then be utilized to synthesize nitrogen containing molecules such as amino acids or nucleic acids. However, ccNiR’s primary role is to help extract energy from the reduction; ammonia is simply a potentially useful byproduct. In general, heterotrophic organisms feed on electron-rich substances such as sugars or fatty acids. During the metabolism of these substances large numbers of electrons are produced. Many organisms use oxygen as the final acceptor of these electrons, in which case water is formed. However, some organisms can use alternative electron acceptors such as nitrite, which is where ccNiR comes in.
| |||||||||||
3D structures of nitric reductase
Cu-containing nitrite reductase with copper only
1nia, 1nib, 1nic, 1nid, 1nie, 1nif, 2nrd, 1kcb, 1rzp, 1rzq, 2bw4, 2bw5, 2avf – AcNIR + Cu – Achromobacter cycloclastes
2afn, 1aq8, 1as7, 2fjs, 2pp7, 2pp8, 3h4h, 3h56 - AfNIR + Cu – Alcaligenes faecalis
1ntd, 1npj, 1npn, 1zdq, 3h4f - AfNIR (mutant) + Cu
1ndr, 1ndt, 1bq5, 1hau, 1haw, 1oe1, 1oe3 - AxNIR + Cu – Achromobacter xylosoxidans
1oe2, 2jfc - AxNIR (mutant) + Cu
1mzz, 2dy2 - RsNIR + Cu – Rhodobacter sphaeroides
2dv6 - NIR + Cu – Hyphomicrobium denitrificans
Cu-containing nitrite reductase with variety of metals
1et5, 1et8 - AfNIR (mutant) + Zn + Cu
1et7 - AfNIR (mutant) + Cd + Cu
2vm3, 2vm4, 2vw4, 2vw6, 2vw7, 2vn3 - AxNIR + Zn + Cu
2bo0 - AxNIR (mutant) + Zn
2vmj - AxNIR + Zn
1gs6 - AxNIR (mutant) + Mg + Cu
1gs7, 1wae, 1wa0, 1wa1, 2bp0, 2bp8, 2xx0, 2xxf, 2xxg - AxNIR (mutant) + Zn + Cu
2zon - AxNIR + heme + Cu
1mzy, 1zv2, 2a3t - RsNIR + Mg + Cu
1n70 - RsNIR (mutant) + Mg + Cu
Cu-containing nitrite reductase binary complex
1as6, 1as8, 1sjm, 2ppc - AfNIR + NO2 + Cu
2e86 - AfNIR + N3 + Cu
1j9q, 1j9r, 1j9s, 1j9t, 1l9o, 1l9p, 1l9q, 1l9r, 1l9s, 1l9t - AfNIR (mutant) + NO2 + Cu
1zds, 2b08 - AfNIR (mutant) + acetamide + Cu
1snr - AfNIR + NO + Cu
2pp9 - AfNIR + NO3 + Cu
2ppa - AfNIR + N2O + Cu
2ppd, 2ppe, 2ppf - AfNIR (mutant) + NO + Cu
2p80 - AfNIR + pseudoazurin + Cu
1nds - AxNIR + NO2 + Cu
2xx1 - AxNIR (mutant) + NO2 + Cu
2xwz - AxNIR + NO + NO2 + Cu
1wa2 - AxNIR (mutant) + NO2 + Zn + Cu
2bwd, 2bwi - AcNIR + NO2 + Cu
2y1a - AcNIR + NO + Cu
2dws, 2dwt - RsNIR + NO2 + Cu
Heme-containing nitrite reductase
1aof, 1qks, 1hj4, 1hj5, 1h9x, 1hcm - PpNIR – Paracoccus pantotrophus
1gq1 - PpNIR (mutant)
1nir, 1bl9, 1n15, 1n50, 1n90 – PaNIR – Pseudomonas aeruginosa
1hzu - PaNIR (mutant)
1qdb - NIR – Sulfurospirillum deleyianum
1fs7, 1fs8 - WsNIR – Wolinella succinogenes
3bng - WsNIR (mutant)
1gu6, 2rdz, 3tor – EcNIR – Escherichia coli
2rf7 - EcNIR (mutant)
2jo6 – EcNIR small subunit – NMR
2jza - NIR small subunit – Pectobacterium atrosepticum - NMR
1oah – NIR – Desulfovibrio desulfuricans
2j7a - DvNIR – Desulfovibrio vulgaris
2ot4, 3gm6, 3fo3, 3sce, 3uu9 - TnNIR – Thioalkalivibrio nitratireducens
Heme-containing nitrite reductase binary complex
1dy7 - PpNIR + CO
1hj3 - PpNIR + O2
2e81 - WsNIR + NH2OH
2vr0 - DvNIR + HQNO inhibitor
Heme-containing nitrite reductase binary complex with cyanide
1h9y - PpNIR + CN
1gjq - PaNIR + CN
1e2r - NIR + CN – Paracoccus denitrificans
Heme-containing nitrite reductase binary complex with nitric oxide
1nno - PaNIR + NO
1hzv - PaNIR (mutant) + NO
1aom, 1aoq - PpNIR + NO + NO2
Heme-containing nitrite reductase binary complex with nitrite
2e80 - WsNIR + NO2
3bnh - WsNIR (mutant) + NO
3d1i, 3rkh, 3owm - TnNIR + NO2
Heme-containing nitrite reductase binary complex with azide
2zo5 - TnNIR + N3
3s7w - TnNIR + NO2 + N3
1fs9 - WsNIR + N3
Heme-containing nitrite reductase binary complex with sulfite
3mmo - WsNIR + SO3
3bnj - WsNIR (mutant) + SO3
3lg1, 3lgq, 3f29, 3ttb - TnNIR + SO3
3l1t - EcNIR + SO3
Siroheme-containing nitrite reductase
2akj - NIR – spinach
3b0g, 3b0h - ToNIR – tobacco
3b0j, 3b0l, 3b0m, 3b0n - ToNIR (mutant)
- ↑ 1.0 1.1 1.2 Youngblut M, Judd ET, Srajer V, Sayyed B, Goelzer T, Elliott SJ, Schmidt M, Pacheco AA. Laue crystal structure of Shewanella oneidensis cytochrome c nitrite reductase from a high-yield expression system. J Biol Inorg Chem. 2012 Mar 2. PMID:22382353 doi:10.1007/s00775-012-0885-0