Sandbox Reserved 508
From Proteopedia
| Line 45: | Line 45: | ||
by nucleotide binding (eg. GDP). Lys141 and Arg185, which are speculated to bind to GDP, are | by nucleotide binding (eg. GDP). Lys141 and Arg185, which are speculated to bind to GDP, are | ||
shown in light sky blue.]] | shown in light sky blue.]] | ||
| + | [[Image:BCSMART_11-12_Figure_6.JPG|right|375px|thumb|'''FIGURE 6: View of UCP2 Down the Barrel''' | ||
| + | <br> | ||
| + | View from matrix side through the putative transport path of UCP2. Our model may support the mechanism | ||
| + | where a protonated fatty acid flips through the inner membrane into the matrix, loses its proton | ||
| + | due to the basic interior environment, and is attracted to the matrix side by positively charged residues | ||
| + | (navy). Additional positively charged residues in the interior of the barrel may pass the fatty acid anion | ||
| + | towards the inter membrane space.]] | ||
Revision as of 22:21, 29 May 2012
Uncoupling Protein 2
Introduction
Ischemic Heart Disease, the underlying cause of myocardial infarctions (heart attacks), is the leading cause of death in the world. Annually 785,000 American alone experience their first heart attack and 470,000 have a second or third. Cardiomyocytes (contracting heart cells) need energy to function optimally. Mitochondria in the cardiomyocytes provide that energy in the form of ATP through the metabolic process of oxidative phosphorylation, specifically the electron transport chain coupled to the enzyme ATP synthase. However, these mitochondria are vulnerable to injury during recovery after heart attack when the cardiomyocytes again receive blood flow (reperfusion) due to the over-production of reactive oxygen species (ROS or oxygen radicals). An excess of oxygen radicals can be very damaging due to their high chemical reactivity, specifically their ability to break chemical bonds in DNA, RNA, and proteins within the mitochondria and in the cell cytoplasm.
Abstract
Heart diseases are the leading cause of death for Americans today. Mitochondria play a crucial role in recovery following ischemia (blood flow restriction) and reperfusion (blood flow return) injury, when a surge of reactive oxygen species (radicals) originating from the mitochondrial electron transport chain causes damage to proteins, lipids and DNA. Uncoupling protein 2 (UCP2), an inner mitochondrial membrane transport protein, is speculated to participate in this protection. The presumed function of UCP2 is carrying protons (H+) into the mitochondrial matrix along a concentration gradient generated by the electron transport chain. Normally, this proton (H+) gradient is used by ATP synthase to phosphorylate ADP to ATP. Under certain conditions, protons (H+) may preferentially be transported through UCP2, creating a detour past ATP synthase (“uncoupling”). Such uncoupling reduces damaging reactive oxygen species whose presence may actually activate UCP2 by residue modification. There are two proposed mechanisms for the transport of protons (H+) into the matrix. One is the direct transport of protons (H+) through UCP2. Alternatively, a fatty acid anion is transported out of the matrix through UCP2, while the protonated fatty acid permeates through the membrane into the matrix. UCP2 must be tightly regulated so it is only active when required, enabling the mitochondria to produce ATP. Understanding transport mechanism and regulation of UCP2 could lead to effective prevention of tissue injury due to heart attack. The Brookfield Central High School SMART Team created a physical model of UCP2 using 3-D modeling printing technology in order to better understand the structure-function relationship of UCP2.
Figures
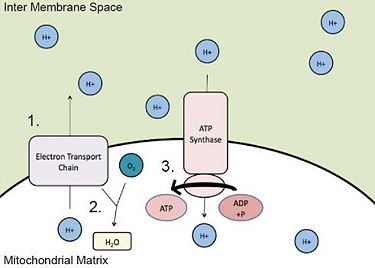
1. The electron transport chain pumps protons (H+) into the inter membrane space.
2. Protons (H+) in the matrix bond with oxygen (O2) to form water (H2O) in a reduction reaction.
3. Protons (H+) from the inter membrane space are used by ATP synthase, to provide energy for the conversion of ADP to ATP.
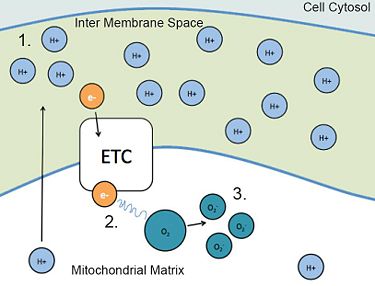
1. During reperfusion (return of blood flow), the electron transport chain sends more protons (H+) into the inter membrane space, producing a high concentration.
2. A high concentration gradient of protons (H+) “backs up” the electrons, allowing them to leave the ETC.
3. The excess electrons in the matrix can bind to oxygen, generating reactive oxygen species (ROS) that carry a negative charge (O2 -)
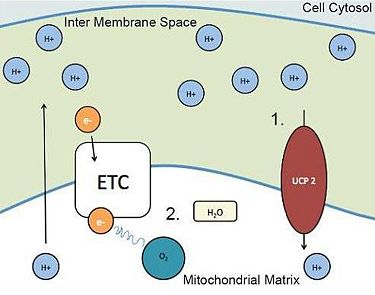
One way the mitochondria can alleviate the proton buildup in the inter membrane space is to transport the protons (H+) back into the matrix. One possible protein involved in this transportation is uncoupling protein 2 (UCP2). 1.UCP2 brings protons (H+) into the matrix, relieving the high concentration gradient.
2.The “back up” of electrons and radical production is reduced, minimizing damage to cardiomyocytes.
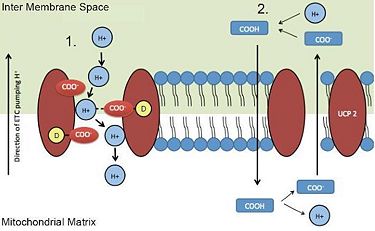
There are two proposed mechanisms for UCP2 transport of protons (H+) into the mitochondria.
1. Direct proton (H+) transport via negatively charged UCP2 residues through the membrane.
2. A neutral fatty acid is flipped through the mitochondrial membrane into the matrix, and because of the basic environment, it releases the proton (H+). The now negatively charged anion is then transported through the membrane by UCP2.
UCP2 is an inner mitochondrial membrane protein consisting of 6 transmembrane helices, shown in orange, with both the N- and C- termini pointing to the inter membrane space. UCP2 activity is inhibited by nucleotide binding (eg. GDP). Lys141 and Arg185, which are speculated to bind to GDP, are shown in light sky blue.
View from matrix side through the putative transport path of UCP2. Our model may support the mechanism where a protonated fatty acid flips through the inner membrane into the matrix, loses its proton due to the basic interior environment, and is attracted to the matrix side by positively charged residues (navy). Additional positively charged residues in the interior of the barrel may pass the fatty acid anion towards the inter membrane space.
