Wheat FKBP73
From Proteopedia
| Line 20: | Line 20: | ||
<StructureSection load='3jym.pdb' size='500' frame='true' align='right' scene='3jym/Cv/2' > | <StructureSection load='3jym.pdb' size='500' frame='true' align='right' scene='3jym/Cv/2' > | ||
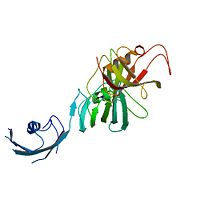
| - | Ribbon representation of the <scene name='3jym/Cv/3'>three FKBP domains</scene>; <font color='blueviolet'><b>wFK73_1 (residues 1–148) in blueviolet</b></font>, <font color='cyan'><b>wFK73_2 (residues 149–266) in cyan</b></font> and <font color='magenta'><b>wFK73_3 (residues 267–386) in magenta</b></font>. The wFK73_1 domain exhibits electron density only between residues 33–38, 54–69 and 87–148. The bulges and the flaps as well as the N- and C-termini are labeled. The three FK506 binding (FK) domains of wFKBP73 are held together mainly by <scene name='3jym/Cv/4'>salt bridge networks</scene> situated between each pair of domains. The wFK73_2-wFK73_1 domains are held by a salt bridge between Lys162–Glu62, and a salt bridge network between Arg151–Asp61 and Glu58. The interface between wFK73_2-wFK73_3 is held by two salt bridges between Lys204–Glu269, and Glu178–Lys279. The interactions Lys162–Glu62 and Glu178–Lys279, involve conserved residues (Glu62 from wFK73_1 and Glu178 from wFK73_2, Lys162 from wFK73_2 and Lys279 from wFK73_3). | + | Ribbon representation of the <scene name='3jym/Cv/3'>three FKBP domains</scene>; <font color='blueviolet'><b>wFK73_1 (residues 1–148) in blueviolet</b></font>, <font color='cyan'><b>wFK73_2 (residues 149–266) in cyan</b></font> and <font color='magenta'><b>wFK73_3 (residues 267–386) in magenta</b></font> ([[3jym]]). The wFK73_1 domain exhibits electron density only between residues 33–38, 54–69 and 87–148. The bulges and the flaps as well as the N- and C-termini are labeled. The three FK506 binding (FK) domains of wFKBP73 are held together mainly by <scene name='3jym/Cv/4'>salt bridge networks</scene> situated between each pair of domains. The wFK73_2-wFK73_1 domains are held by a salt bridge between Lys162–Glu62, and a salt bridge network between Arg151–Asp61 and Glu58. The interface between wFK73_2-wFK73_3 is held by two salt bridges between Lys204–Glu269, and Glu178–Lys279. The interactions Lys162–Glu62 and Glu178–Lys279, involve conserved residues (Glu62 from wFK73_1 and Glu178 from wFK73_2, Lys162 from wFK73_2 and Lys279 from wFK73_3). |
{{Clear}} | {{Clear}} | ||
Revision as of 08:56, 5 June 2012
Crystal Structure of the 3 FKBP domains of wheat FKBP73
(see also FK506 binding protein)
Here we describe the crystal structure of the N-terminal domain of the FK506-binding protein (FKBP) from wheat (wFKBP73), which is the first structure presenting three FK domains (wFK73_1, wFK73_2 and wFK73_3). The crystal model includes wFK73_2 and wFK73_3 domains and only part of the wFK73_1 domain. The wFK73_1 domain is responsible for binding FK506 and for peptidyl prolyl cis/trans isomerase (PPIase) activity, while the wFK73_2 and wFK73_3 domains lack these activities. A structure-based sequence comparison demonstrated that the absence of a large enough hydrophobic pocket important for PPIase activity, and of the conserved residues necessary for drug binding in the wFK73_2 and wFK73_3 domains explains the lack of these activities in these domains. Sequence and structural comparison between the three wFKBP73 domains suggest that the wFK73_2 domain is the most divergent. A structural comparison of the FK domains of wFKBP73 with other FKBPs containing more than one FK domain, revealed that while the overall architecture of each of the three FK domains displays a typical FKBP fold, their relative arrangement in space is unique and may have important functional implications. We suggest that the existence of FKBPs with three FK domains offers additional interactive options for these plant proteins enlarging the overall regulatory functions of these proteins.
Crystal structure of the three FK506 binding protein domains of wheat FKBP73: evidence for a unique wFK73_2 domain., Unger T, Dym O, Albeck S, Jacobovitch Y, Bernehim R, Marom D, Pisanty O, Breiman A, J Struct Funct Genomics. 2010 Jun;11(2):113-23. Epub 2010 Mar 20. PMID:20306145
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
| |||||||||||
About this Structure
3JYM is a 2 chains structure with sequences from Triticum aestivum. Full crystallographic information is available from OCA.
Reference
- Unger T, Dym O, Albeck S, Jacobovitch Y, Bernehim R, Marom D, Pisanty O, Breiman A. Crystal structure of the three FK506 binding protein domains of wheat FKBP73: evidence for a unique wFK73_2 domain. J Struct Funct Genomics. 2010 Jun;11(2):113-23. Epub 2010 Mar 20. PMID:20306145 doi:10.1007/s10969-010-9085-8