Sandbox SouthUniversity1
From Proteopedia
| Line 40: | Line 40: | ||
|| Feedback for distractor. | || Feedback for distractor. | ||
</quiz> | </quiz> | ||
| + | |||
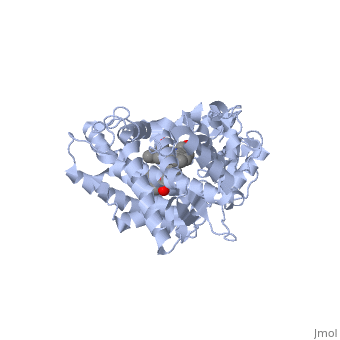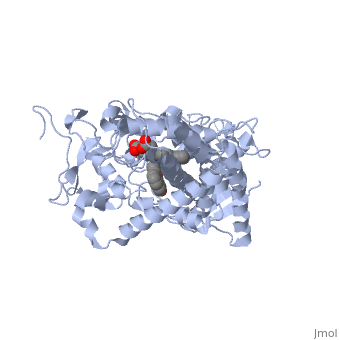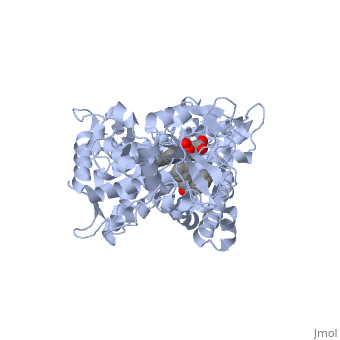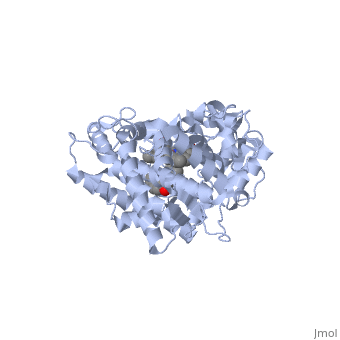
| + | The substrate (flavone) shown here is different from many other substrates of this CYP. Besides being a substrate of CYP1A2, it is also an inhibitor. It inhibits the metabolism of other CYP1A2 substrates because it binds very tightly to this enzyme. Thus, while the CYP is bound, it is unavailable to metabolize other substrates (e.g. drugs). Therefor, if a drug were to be coadministered with this compound, it would not be broken down to the extent expected, and could build up to toxic levels. | ||
| + | |||
| + | The reason that this flavone is bound so tightly to the enzyme is that it's shape and electron density are complementary to the binding pocket. This is examined <scene name='Sandbox_SouthUniversity1/2hi4_blue_transparent_ribbons/1'>next.</scene> First examine the shape of the flavone bound to the protein. Next, the Van derWaals surface of the cavity is <scene name='Sandbox_SouthUniversity1/displayed./1'>TextToBeDisplayed</scene> | ||
Different members of the CYP450 enzymes preferentially metabolize different xenobiotics. What features of a drug do you think might cause it to be metabolized by one CYP versus another? | Different members of the CYP450 enzymes preferentially metabolize different xenobiotics. What features of a drug do you think might cause it to be metabolized by one CYP versus another? | ||
| + | |||
| + | |||
| + | |||
| + | |||
Draft: <illustration of residues in active site> | Draft: <illustration of residues in active site> | ||
Draft animation <scene name='Sandbox_SouthUniversity1/Act_residues_shown/1'>Highlighting residues around ligand</scene> | Draft animation <scene name='Sandbox_SouthUniversity1/Act_residues_shown/1'>Highlighting residues around ligand</scene> | ||
Revision as of 17:27, 6 June 2012
==Selectivity of Drug Metabolism by Cytochrome P450 Enzymes==
| |||||||||||
The substrate (flavone) shown here is different from many other substrates of this CYP. Besides being a substrate of CYP1A2, it is also an inhibitor. It inhibits the metabolism of other CYP1A2 substrates because it binds very tightly to this enzyme. Thus, while the CYP is bound, it is unavailable to metabolize other substrates (e.g. drugs). Therefor, if a drug were to be coadministered with this compound, it would not be broken down to the extent expected, and could build up to toxic levels.
The reason that this flavone is bound so tightly to the enzyme is that it's shape and electron density are complementary to the binding pocket. This is examined First examine the shape of the flavone bound to the protein. Next, the Van derWaals surface of the cavity is
Different members of the CYP450 enzymes preferentially metabolize different xenobiotics. What features of a drug do you think might cause it to be metabolized by one CYP versus another?
Draft: <illustration of residues in active site>
Draft animation
Draft animation
draft: <insert quiz here on electrostatic and steric factors>
draft: <insert animations here contrasting the shape of the active site of CYP3A4 and e.g. CYP2E1>
draft: <insert examples of substrates for 2 different CYPs, overlay them on top of each other illustrate the molecular commonalities>
draft <illustrate the size of the binding pockets and their electronic character>
draft test of scene names:
<Draft Quiz>
References The structure of CYP1A2 shown above is found in the Research Collaboration of Structural Bioinformatics Protein Database as entry 2HI4.