Acetylcholinesterase inhibited by nerve agent soman
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | [[Image:1som.jpg|left| | + | <StructureSection load="1som" size="400" color="" frame="true" spin="on" Scene ="1som/Ache_soman/1" align="right" caption > |
| + | |||
| + | [[Image:1som.jpg|left|200px]]<br /> | ||
| - | {{STRUCTURE_1som | PDB=1som | SCENE= }} | ||
'''Acetylcholinesterase inhibited by nerve agent soman'''<br /> | '''Acetylcholinesterase inhibited by nerve agent soman'''<br /> | ||
| Line 12: | Line 13: | ||
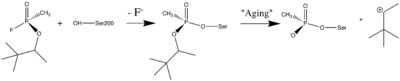
[http://en.wikipedia.org/wiki/Organophosphorus Organophosphorus] (OP)[http://en.wikipedia.org/wiki/Acid_anhydride acid anhydride] [http://en.wikipedia.org/wiki/Nerve_agent nerve agents] are potent inhibitors which rapidly phosphonylate AChE and then may undergo an internal dealkylation reaction (called "aging") to produce an OP-enzyme conjugate that cannot be reactivated. | [http://en.wikipedia.org/wiki/Organophosphorus Organophosphorus] (OP)[http://en.wikipedia.org/wiki/Acid_anhydride acid anhydride] [http://en.wikipedia.org/wiki/Nerve_agent nerve agents] are potent inhibitors which rapidly phosphonylate AChE and then may undergo an internal dealkylation reaction (called "aging") to produce an OP-enzyme conjugate that cannot be reactivated. | ||
| - | [[Image:Soman_reaction.png | left | thumb | | + | [[Image:Soman_reaction.png | left | thumb | 400px | Reaction between Ser200OG and Soman, assuming an in-line attack by the OG, followed by spontaneous dealkylation of the O-pinacolyl group.]] |
| - | + | ||
| - | + | ||
| - | + | ||
To understand the basis for [http://en.wikipedia.org/wiki/Enzyme_inhibitor#Irreversible_inhibitors irreversible inhibition], we solved the structure of the aged conjugate obtained by reaction of ''Torpedo californica'' AChE (''Tc''AChE) with [http://en.wikipedia.org/wiki/Soman O-pinacolylmethylphosphonofluoridate] (soman) by X-ray crystallography to 2.2Å resolution. The highest positive difference density peak corresponded to the OP phosphorus and was located within covalent bonding distance of the active-site serine (S200). The <scene name='1som/Soman_active_site/3'>OP-oxygen atoms</scene> are within hydrogen-bonding distance of four potential donors from catalytic subsites of the enzyme, suggesting that electrostatic forces significantly stabilize the aged enzyme. The methyl group of soman occupies the <scene name='1som/Soman_acyl_binding/2'>acyl binding pocket</scene>, bounded by Trp233, Phe288, and Phe290. The active sites of aged sarin-TcAChE ([[1cfj]]) and soman-TcAChE were essentially identical and provided structural models for the negatively charged, tetrahedral intermediate that occurs during deacylation with the natural substrate, acetylcholine. | To understand the basis for [http://en.wikipedia.org/wiki/Enzyme_inhibitor#Irreversible_inhibitors irreversible inhibition], we solved the structure of the aged conjugate obtained by reaction of ''Torpedo californica'' AChE (''Tc''AChE) with [http://en.wikipedia.org/wiki/Soman O-pinacolylmethylphosphonofluoridate] (soman) by X-ray crystallography to 2.2Å resolution. The highest positive difference density peak corresponded to the OP phosphorus and was located within covalent bonding distance of the active-site serine (S200). The <scene name='1som/Soman_active_site/3'>OP-oxygen atoms</scene> are within hydrogen-bonding distance of four potential donors from catalytic subsites of the enzyme, suggesting that electrostatic forces significantly stabilize the aged enzyme. The methyl group of soman occupies the <scene name='1som/Soman_acyl_binding/2'>acyl binding pocket</scene>, bounded by Trp233, Phe288, and Phe290. The active sites of aged sarin-TcAChE ([[1cfj]]) and soman-TcAChE were essentially identical and provided structural models for the negatively charged, tetrahedral intermediate that occurs during deacylation with the natural substrate, acetylcholine. | ||
| Line 24: | Line 22: | ||
For additional information, see: [[Alzheimer's Disease]] | For additional information, see: [[Alzheimer's Disease]] | ||
<br /> | <br /> | ||
| - | + | </StructureSection> | |
==Reference== | ==Reference== | ||
Crystal structures of aged phosphonylated acetylcholinesterase: nerve agent reaction products at the atomic level., Millard CB, Kryger G, Ordentlich A, Greenblatt HM, Harel M, Raves ML, Segall Y, Barak D, Shafferman A, Silman I, Sussman JL, Biochemistry. 1999 Jun 1;38(22):7032-9. PMID:[http://ispc.weizmann.ac.il//pmbin/getpm?pmid=10353814 10353814] | Crystal structures of aged phosphonylated acetylcholinesterase: nerve agent reaction products at the atomic level., Millard CB, Kryger G, Ordentlich A, Greenblatt HM, Harel M, Raves ML, Segall Y, Barak D, Shafferman A, Silman I, Sussman JL, Biochemistry. 1999 Jun 1;38(22):7032-9. PMID:[http://ispc.weizmann.ac.il//pmbin/getpm?pmid=10353814 10353814] | ||
Revision as of 08:45, 10 June 2012
| |||||||||||
Reference
Crystal structures of aged phosphonylated acetylcholinesterase: nerve agent reaction products at the atomic level., Millard CB, Kryger G, Ordentlich A, Greenblatt HM, Harel M, Raves ML, Segall Y, Barak D, Shafferman A, Silman I, Sussman JL, Biochemistry. 1999 Jun 1;38(22):7032-9. PMID:10353814 Created with the participation of Harry Greenblatt, Jaime Prilusky, Joel L. Sussman, and David Canner.