2nvb
From Proteopedia
| Line 1: | Line 1: | ||
[[Image:2nvb.png|left|200px]] | [[Image:2nvb.png|left|200px]] | ||
| - | <!-- | ||
| - | The line below this paragraph, containing "STRUCTURE_2nvb", creates the "Structure Box" on the page. | ||
| - | You may change the PDB parameter (which sets the PDB file loaded into the applet) | ||
| - | or the SCENE parameter (which sets the initial scene displayed when the page is loaded), | ||
| - | or leave the SCENE parameter empty for the default display. | ||
| - | --> | ||
{{STRUCTURE_2nvb| PDB=2nvb | SCENE= }} | {{STRUCTURE_2nvb| PDB=2nvb | SCENE= }} | ||
===Contribution of Pro275 to the Thermostability of the Alcohol Dehydrogenases (ADHs)=== | ===Contribution of Pro275 to the Thermostability of the Alcohol Dehydrogenases (ADHs)=== | ||
| - | |||
| - | <!-- | ||
| - | The line below this paragraph, {{ABSTRACT_PUBMED_18260103}}, adds the Publication Abstract to the page | ||
| - | (as it appears on PubMed at http://www.pubmed.gov), where 18260103 is the PubMed ID number. | ||
| - | --> | ||
{{ABSTRACT_PUBMED_18260103}} | {{ABSTRACT_PUBMED_18260103}} | ||
| Line 22: | Line 11: | ||
==See Also== | ==See Also== | ||
| - | *[[Alcohol Dehydrogenase from Entamoeba histolotica|Alcohol Dehydrogenase from Entamoeba histolotica]] | ||
*[[Alcohol dehydrogenase|Alcohol dehydrogenase]] | *[[Alcohol dehydrogenase|Alcohol dehydrogenase]] | ||
| + | *[[Alcohol dehydrogenase from Entamoeba histolytica|Alcohol dehydrogenase from Entamoeba histolytica]] | ||
*[[Contribution of Pro275 to the Thermostability of the Alcohol Dehydrogenases|Contribution of Pro275 to the Thermostability of the Alcohol Dehydrogenases]] | *[[Contribution of Pro275 to the Thermostability of the Alcohol Dehydrogenases|Contribution of Pro275 to the Thermostability of the Alcohol Dehydrogenases]] | ||
*[[D275P mutant of alcohol dehydrogenase from protozoa Entamoeba histolytica|D275P mutant of alcohol dehydrogenase from protozoa Entamoeba histolytica]] | *[[D275P mutant of alcohol dehydrogenase from protozoa Entamoeba histolytica|D275P mutant of alcohol dehydrogenase from protozoa Entamoeba histolytica]] | ||
Revision as of 10:17, 15 July 2012
Contents |
Contribution of Pro275 to the Thermostability of the Alcohol Dehydrogenases (ADHs)
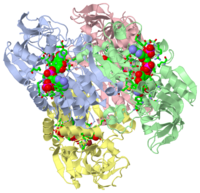
Analysis of the three-dimensional structures of two closely related thermophilic and hyperthermophilic alcohol dehydrogenases (ADHs) from the respective microorganisms Entamoeba histolytica (EhADH1) and Thermoanaerobacter brockii (TbADH) suggested that a unique, strategically located proline residue (Pro275) at the center of the dimerization interface might be crucial for maintaining the thermal stability of TbADH. To assess the contribution of Pro275 to the thermal stability of the ADHs, we applied site-directed mutagenesis to replace Asp275 of EhADH1 with Pro (D275P-EhADH1) and conversely Pro275 of TbADH with Asp (P275D-TbADH). The results indicate that replacing Asp275 with Pro significantly enhances the thermal stability of EhADH1 (DeltaT(1/2) </= +10 degrees C), whereas the reverse mutation in the thermophilic TbADH (P275D-TbADH) reduces the thermostability of the enzyme (DeltaT(1/2) </= -18.8 degrees C). Analysis of the crystal structures of the thermostabilized mutant D275P-EhADH1 and the thermocompromised mutant P275D-TbADH suggest that a proline residue at position 275 thermostabilized the enzymes by reducing flexibility and by reinforcing hydrophobic interactions at the dimer-dimer interface of the tetrameric ADHs. Proteins 2008. (c) 2008 Wiley-Liss, Inc.
Thermal stabilization of the protozoan Entamoeba histolytica alcohol dehydrogenase by a single proline substitution., Goihberg E, Dym O, Tel-Or S, Shimon L, Frolow F, Peretz M, Burstein Y, Proteins. 2008 Feb 7;. PMID:18260103
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
About this Structure
2nvb is a 4 chain structure of Alcohol dehydrogenase with sequence from Thermoanaerobacter brockii. Full crystallographic information is available from OCA.
See Also
- Alcohol dehydrogenase
- Alcohol dehydrogenase from Entamoeba histolytica
- Contribution of Pro275 to the Thermostability of the Alcohol Dehydrogenases
- D275P mutant of alcohol dehydrogenase from protozoa Entamoeba histolytica
- Tetrameric alcohol dehydrogenases
Reference
- Goihberg E, Dym O, Tel-Or S, Shimon L, Frolow F, Peretz M, Burstein Y. Thermal stabilization of the protozoan Entamoeba histolytica alcohol dehydrogenase by a single proline substitution. Proteins. 2008 Feb 7;. PMID:18260103 doi:10.1002/prot.21946