1a0a
From Proteopedia
(New page: 200px<br /><applet load="1a0a" size="450" color="white" frame="true" align="right" spinBox="true" caption="1a0a, resolution 2.800Å" /> '''PHOSPHATE SYSTEM PO...) |
|||
| Line 1: | Line 1: | ||
| - | [[Image:1a0a.gif|left|200px]]<br /><applet load="1a0a" size=" | + | [[Image:1a0a.gif|left|200px]]<br /><applet load="1a0a" size="350" color="white" frame="true" align="right" spinBox="true" |
caption="1a0a, resolution 2.800Å" /> | caption="1a0a, resolution 2.800Å" /> | ||
'''PHOSPHATE SYSTEM POSITIVE REGULATORY PROTEIN PHO4/DNA COMPLEX'''<br /> | '''PHOSPHATE SYSTEM POSITIVE REGULATORY PROTEIN PHO4/DNA COMPLEX'''<br /> | ||
==Overview== | ==Overview== | ||
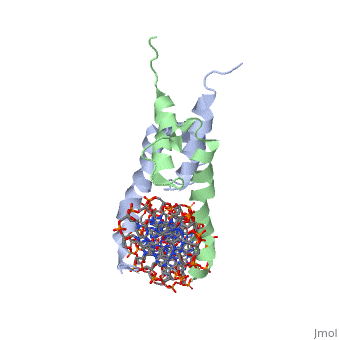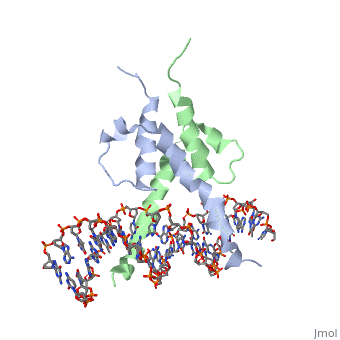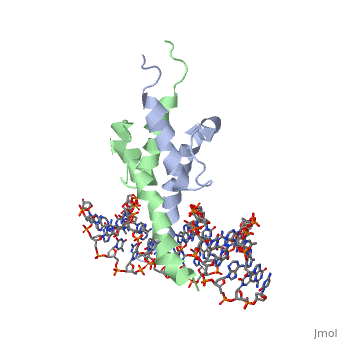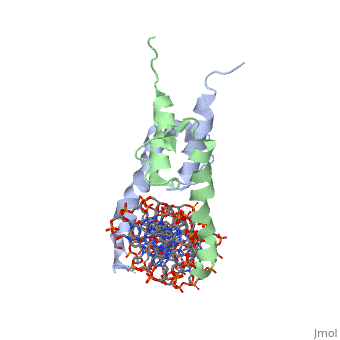
| - | The crystal structure of a DNA-binding domain of PHO4 complexed with DNA | + | The crystal structure of a DNA-binding domain of PHO4 complexed with DNA at 2.8 A resolution revealed that the domain folds into a basic-helix-loop-helix (bHLH) motif with a long but compact loop that contains a short alpha-helical segment. This helical structure positions a tryptophan residue into an aromatic cluster so as to make the loop compact. PHO4 binds to DNA as a homodimer with direct reading of both the core E-box sequence CACGTG and its 3'-flanking bases. The 3'-flanking bases GG are recognized by Arg2 and His5. The residues involved in the E-box recognition are His5, Glu9 and Arg13, as already reported for bHLH/Zip proteins MAX and USF, and are different from those recognized by bHLH proteins MyoD and E47, although PHO4 is a bHLH protein. |
==About this Structure== | ==About this Structure== | ||
| - | 1A0A is a [http://en.wikipedia.org/wiki/Single_protein Single protein] structure of sequence from [http://en.wikipedia.org/wiki/Saccharomyces_cerevisiae Saccharomyces cerevisiae]. Full crystallographic information is available from [http:// | + | 1A0A is a [http://en.wikipedia.org/wiki/Single_protein Single protein] structure of sequence from [http://en.wikipedia.org/wiki/Saccharomyces_cerevisiae Saccharomyces cerevisiae]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1A0A OCA]. |
==Reference== | ==Reference== | ||
| Line 25: | Line 25: | ||
[[Category: transcription factor]] | [[Category: transcription factor]] | ||
| - | ''Page seeded by [http:// | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Feb 21 11:39:29 2008'' |
Revision as of 09:39, 21 February 2008
|
PHOSPHATE SYSTEM POSITIVE REGULATORY PROTEIN PHO4/DNA COMPLEX
Overview
The crystal structure of a DNA-binding domain of PHO4 complexed with DNA at 2.8 A resolution revealed that the domain folds into a basic-helix-loop-helix (bHLH) motif with a long but compact loop that contains a short alpha-helical segment. This helical structure positions a tryptophan residue into an aromatic cluster so as to make the loop compact. PHO4 binds to DNA as a homodimer with direct reading of both the core E-box sequence CACGTG and its 3'-flanking bases. The 3'-flanking bases GG are recognized by Arg2 and His5. The residues involved in the E-box recognition are His5, Glu9 and Arg13, as already reported for bHLH/Zip proteins MAX and USF, and are different from those recognized by bHLH proteins MyoD and E47, although PHO4 is a bHLH protein.
About this Structure
1A0A is a Single protein structure of sequence from Saccharomyces cerevisiae. Full crystallographic information is available from OCA.
Reference
Crystal structure of PHO4 bHLH domain-DNA complex: flanking base recognition., Shimizu T, Toumoto A, Ihara K, Shimizu M, Kyogoku Y, Ogawa N, Oshima Y, Hakoshima T, EMBO J. 1997 Aug 1;16(15):4689-97. PMID:9303313
Page seeded by OCA on Thu Feb 21 11:39:29 2008