1bh0
From Proteopedia
(New page: 200px<br /> <applet load="1bh0" size="450" color="white" frame="true" align="right" spinBox="true" caption="1bh0, resolution 3.0Å" /> '''STRUCTURE OF A GLUCA...) |
|||
| Line 1: | Line 1: | ||
| - | [[Image:1bh0.gif|left|200px]]<br /> | + | [[Image:1bh0.gif|left|200px]]<br /><applet load="1bh0" size="350" color="white" frame="true" align="right" spinBox="true" |
| - | <applet load="1bh0" size=" | + | |
caption="1bh0, resolution 3.0Å" /> | caption="1bh0, resolution 3.0Å" /> | ||
'''STRUCTURE OF A GLUCAGON ANALOG'''<br /> | '''STRUCTURE OF A GLUCAGON ANALOG'''<br /> | ||
==Overview== | ==Overview== | ||
| - | We have designed and synthesized eight compounds 2-9 which incorporate | + | We have designed and synthesized eight compounds 2-9 which incorporate various amino acid residues in positions 17, 18, and 21 of the glucagon molecule: 2, [Lys17]glucagon amide; 3, [Lys18]glucagon amide; 4, [Nle17,Lys18,Glu21]glucagon amide; 5, [Orn17,18, Glu21]glucagon amide; 6, [d-Arg17]glucagon; 7, [d-Arg18]glucagon; 8, [d-Phe17]glucagon; and 9, [d-Phe18]glucagon. Compared to glucagon (IC50 = 1.5 nM), analogues 2-9 were found to have binding affinity IC50 values (in nM) of 0.7, 4.1, 1.0, 2.0, 5.0, 25.0, 43.0, and 32.0, respectively. When these compounds were tested for their ability to stimulate adenylate cyclase (AC) activity, they were found to be full or partial agonists having maximum stimulation values of 100, 100, 100, 100, 87, 78, 94, and 100%, respectively. On the basis of the X-ray crystal structure of [Lys17,18,Glu21]glucagon amide reported here, the ability to form a salt bridge between Lys18 and Glu21 is probably key to their increased binding and second messenger activities. Among the eight analogues synthesized here, only analogue 4 preserves the ability to form a salt bridge between Lys18 and Glu21. However, since these modifications are minor they do not seem to change the amphiphilic character of the C-terminus, allowing these analogues to reach 78-100% stimulation in the adenylate cyclase assay. Biological data from analogues 6-9 supports the idea that position 18 of glucagon may influence binding only, while position 17 may influence both receptor recognition and transduction. |
==Disease== | ==Disease== | ||
| Line 11: | Line 10: | ||
==About this Structure== | ==About this Structure== | ||
| - | 1BH0 is a [http://en.wikipedia.org/wiki/Single_protein Single protein] structure of sequence from [http://en.wikipedia.org/wiki/ ]. Full crystallographic information is available from [http:// | + | 1BH0 is a [http://en.wikipedia.org/wiki/Single_protein Single protein] structure of sequence from [http://en.wikipedia.org/wiki/ ]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1BH0 OCA]. |
==Reference== | ==Reference== | ||
Structure-function studies on positions 17, 18, and 21 replacement analogues of glucagon: the importance of charged residues and salt bridges in glucagon biological activity., Sturm NS, Lin Y, Burley SK, Krstenansky JL, Ahn JM, Azizeh BY, Trivedi D, Hruby VJ, J Med Chem. 1998 Jul 16;41(15):2693-700. PMID:[http://ispc.weizmann.ac.il//pmbin/getpm?pmid=9667960 9667960] | Structure-function studies on positions 17, 18, and 21 replacement analogues of glucagon: the importance of charged residues and salt bridges in glucagon biological activity., Sturm NS, Lin Y, Burley SK, Krstenansky JL, Ahn JM, Azizeh BY, Trivedi D, Hruby VJ, J Med Chem. 1998 Jul 16;41(15):2693-700. PMID:[http://ispc.weizmann.ac.il//pmbin/getpm?pmid=9667960 9667960] | ||
[[Category: Single protein]] | [[Category: Single protein]] | ||
| - | [[Category: Ahn, J | + | [[Category: Ahn, J M.]] |
| - | [[Category: Azizeh, B | + | [[Category: Azizeh, B Y.]] |
| - | [[Category: Burley, S | + | [[Category: Burley, S K.]] |
| - | [[Category: Hruby, V | + | [[Category: Hruby, V J.]] |
| - | [[Category: Krstenansky, J | + | [[Category: Krstenansky, J L.]] |
[[Category: Lin, Y.]] | [[Category: Lin, Y.]] | ||
| - | [[Category: Sturm, N | + | [[Category: Sturm, N S.]] |
[[Category: Trivedi, D.]] | [[Category: Trivedi, D.]] | ||
[[Category: synthetic hormone]] | [[Category: synthetic hormone]] | ||
| - | ''Page seeded by [http:// | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Feb 21 11:55:13 2008'' |
Revision as of 09:55, 21 February 2008
|
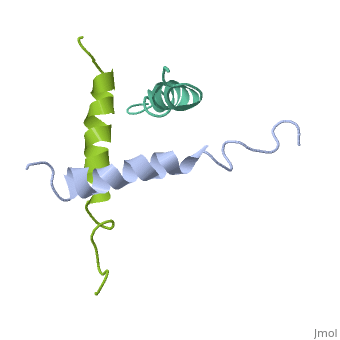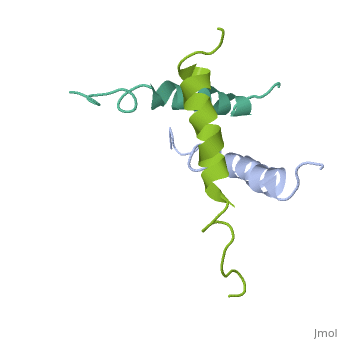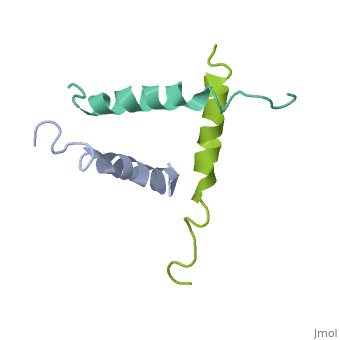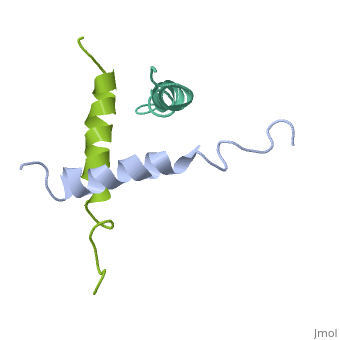
STRUCTURE OF A GLUCAGON ANALOG
Contents |
Overview
We have designed and synthesized eight compounds 2-9 which incorporate various amino acid residues in positions 17, 18, and 21 of the glucagon molecule: 2, [Lys17]glucagon amide; 3, [Lys18]glucagon amide; 4, [Nle17,Lys18,Glu21]glucagon amide; 5, [Orn17,18, Glu21]glucagon amide; 6, [d-Arg17]glucagon; 7, [d-Arg18]glucagon; 8, [d-Phe17]glucagon; and 9, [d-Phe18]glucagon. Compared to glucagon (IC50 = 1.5 nM), analogues 2-9 were found to have binding affinity IC50 values (in nM) of 0.7, 4.1, 1.0, 2.0, 5.0, 25.0, 43.0, and 32.0, respectively. When these compounds were tested for their ability to stimulate adenylate cyclase (AC) activity, they were found to be full or partial agonists having maximum stimulation values of 100, 100, 100, 100, 87, 78, 94, and 100%, respectively. On the basis of the X-ray crystal structure of [Lys17,18,Glu21]glucagon amide reported here, the ability to form a salt bridge between Lys18 and Glu21 is probably key to their increased binding and second messenger activities. Among the eight analogues synthesized here, only analogue 4 preserves the ability to form a salt bridge between Lys18 and Glu21. However, since these modifications are minor they do not seem to change the amphiphilic character of the C-terminus, allowing these analogues to reach 78-100% stimulation in the adenylate cyclase assay. Biological data from analogues 6-9 supports the idea that position 18 of glucagon may influence binding only, while position 17 may influence both receptor recognition and transduction.
Disease
Known disease associated with this structure: Hyperproglucagonemia OMIM:[138030]
About this Structure
1BH0 is a Single protein structure of sequence from [1]. Full crystallographic information is available from OCA.
Reference
Structure-function studies on positions 17, 18, and 21 replacement analogues of glucagon: the importance of charged residues and salt bridges in glucagon biological activity., Sturm NS, Lin Y, Burley SK, Krstenansky JL, Ahn JM, Azizeh BY, Trivedi D, Hruby VJ, J Med Chem. 1998 Jul 16;41(15):2693-700. PMID:9667960
Page seeded by OCA on Thu Feb 21 11:55:13 2008