1f8a
From Proteopedia
(New page: 200px<br /> <applet load="1f8a" size="450" color="white" frame="true" align="right" spinBox="true" caption="1f8a, resolution 1.84Å" /> '''STRUCTURAL BASIS FO...) |
|||
| Line 1: | Line 1: | ||
| - | [[Image:1f8a.gif|left|200px]]<br /> | + | [[Image:1f8a.gif|left|200px]]<br /><applet load="1f8a" size="350" color="white" frame="true" align="right" spinBox="true" |
| - | <applet load="1f8a" size=" | + | |
caption="1f8a, resolution 1.84Å" /> | caption="1f8a, resolution 1.84Å" /> | ||
'''STRUCTURAL BASIS FOR THE PHOSPHOSERINE-PROLINE RECOGNITION BY GROUP IV WW DOMAINS'''<br /> | '''STRUCTURAL BASIS FOR THE PHOSPHOSERINE-PROLINE RECOGNITION BY GROUP IV WW DOMAINS'''<br /> | ||
==Overview== | ==Overview== | ||
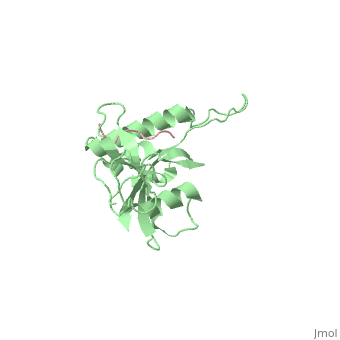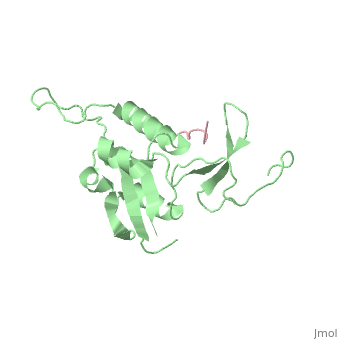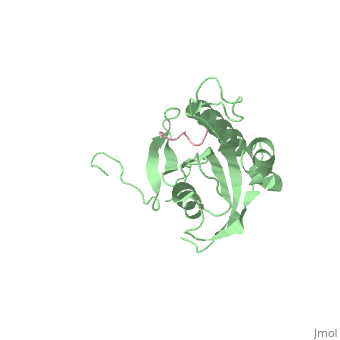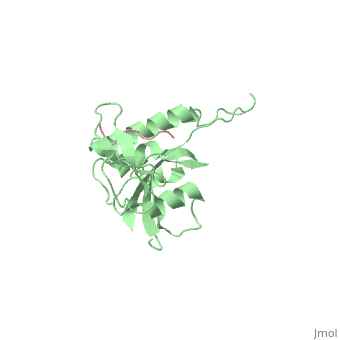
| - | Pin1 contains an N-terminal WW domain and a C-terminal peptidyl-prolyl | + | Pin1 contains an N-terminal WW domain and a C-terminal peptidyl-prolyl cis-trans isomerase (PPIase) domain connected by a flexible linker. To address the energetic and structural basis for WW domain recognition of phosphoserine (P.Ser)/phosphothreonine (P. Thr)- proline containing proteins, we report the energetic and structural analysis of a Pin1-phosphopeptide complex. The X-ray crystal structure of Pin1 bound to a doubly phosphorylated peptide (Tyr-P.Ser-Pro-Thr-P.Ser-Pro-Ser) representing a heptad repeat of the RNA polymerase II large subunit's C-terminal domain (CTD), reveals the residues involved in the recognition of a single P.Ser side chain, the rings of two prolines, and the backbone of the CTD peptide. The side chains of neighboring Arg and Ser residues along with a backbone amide contribute to recognition of P.Ser. The lack of widespread conservation of the Arg and Ser residues responsible for P.Ser recognition in the WW domain family suggests that only a subset of WW domains can bind P.Ser-Pro in a similar fashion to that of Pin1. |
==About this Structure== | ==About this Structure== | ||
| - | 1F8A is a [http://en.wikipedia.org/wiki/Single_protein Single protein] structure of sequence from [http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Active as [http://en.wikipedia.org/wiki/Peptidylprolyl_isomerase Peptidylprolyl isomerase], with EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=5.2.1.8 5.2.1.8] Full crystallographic information is available from [http:// | + | 1F8A is a [http://en.wikipedia.org/wiki/Single_protein Single protein] structure of sequence from [http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Active as [http://en.wikipedia.org/wiki/Peptidylprolyl_isomerase Peptidylprolyl isomerase], with EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=5.2.1.8 5.2.1.8] Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1F8A OCA]. |
==Reference== | ==Reference== | ||
| Line 15: | Line 14: | ||
[[Category: Peptidylprolyl isomerase]] | [[Category: Peptidylprolyl isomerase]] | ||
[[Category: Single protein]] | [[Category: Single protein]] | ||
| - | [[Category: Bowman, M | + | [[Category: Bowman, M E.]] |
[[Category: Hunter, T.]] | [[Category: Hunter, T.]] | ||
| - | [[Category: Lu, K | + | [[Category: Lu, K P.]] |
| - | [[Category: Noel, J | + | [[Category: Noel, J P.]] |
| - | [[Category: Verdecia, M | + | [[Category: Verdecia, M A.]] |
[[Category: peptidyl-proline isomerase]] | [[Category: peptidyl-proline isomerase]] | ||
[[Category: phosphoserine binding]] | [[Category: phosphoserine binding]] | ||
[[Category: ww domain]] | [[Category: ww domain]] | ||
| - | ''Page seeded by [http:// | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Feb 21 12:36:02 2008'' |
Revision as of 10:36, 21 February 2008
|
STRUCTURAL BASIS FOR THE PHOSPHOSERINE-PROLINE RECOGNITION BY GROUP IV WW DOMAINS
Overview
Pin1 contains an N-terminal WW domain and a C-terminal peptidyl-prolyl cis-trans isomerase (PPIase) domain connected by a flexible linker. To address the energetic and structural basis for WW domain recognition of phosphoserine (P.Ser)/phosphothreonine (P. Thr)- proline containing proteins, we report the energetic and structural analysis of a Pin1-phosphopeptide complex. The X-ray crystal structure of Pin1 bound to a doubly phosphorylated peptide (Tyr-P.Ser-Pro-Thr-P.Ser-Pro-Ser) representing a heptad repeat of the RNA polymerase II large subunit's C-terminal domain (CTD), reveals the residues involved in the recognition of a single P.Ser side chain, the rings of two prolines, and the backbone of the CTD peptide. The side chains of neighboring Arg and Ser residues along with a backbone amide contribute to recognition of P.Ser. The lack of widespread conservation of the Arg and Ser residues responsible for P.Ser recognition in the WW domain family suggests that only a subset of WW domains can bind P.Ser-Pro in a similar fashion to that of Pin1.
About this Structure
1F8A is a Single protein structure of sequence from Homo sapiens. Active as Peptidylprolyl isomerase, with EC number 5.2.1.8 Full crystallographic information is available from OCA.
Reference
Structural basis for phosphoserine-proline recognition by group IV WW domains., Verdecia MA, Bowman ME, Lu KP, Hunter T, Noel JP, Nat Struct Biol. 2000 Aug;7(8):639-43. PMID:10932246
Page seeded by OCA on Thu Feb 21 12:36:02 2008