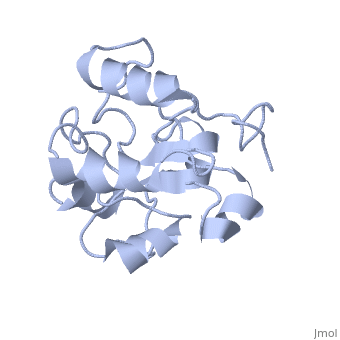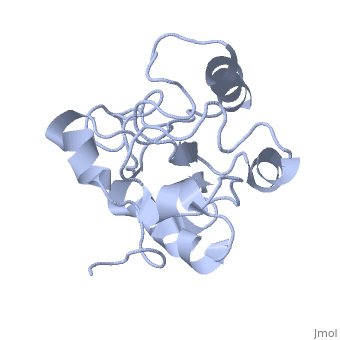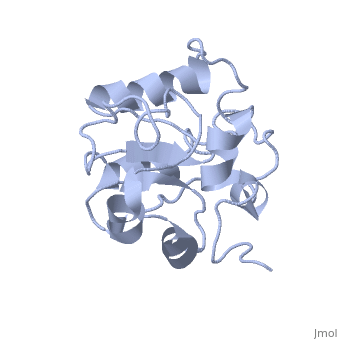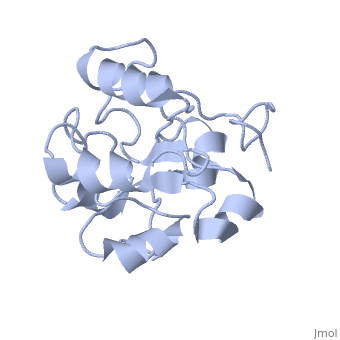1hzm
From Proteopedia
| Line 4: | Line 4: | ||
==Overview== | ==Overview== | ||
| - | MAP kinases (MAPKs), which control mitogenic signal transduction in all | + | MAP kinases (MAPKs), which control mitogenic signal transduction in all eukaryotic organisms, are inactivated by dual specificity MAPK phosphatases (MKPs). MKP-3, a prototypical MKP, achieves substrate specificity through its N-terminal domain binding to the MAPK ERK2, resulting in the activation of its C-terminal phosphatase domain. The solution structure and biochemical analysis of the ERK2 binding (EB) domain of MKP-3 show that regions that are essential for ERK2 binding partly overlap with its sites that interact with the C-terminal catalytic domain, and that these interactions are functionally coupled to the active site residues of MKP-3. Our findings suggest a novel mechanism by which the EB domain binding to ERK2 is transduced to cause a conformational change of the C-terminal catalytic domain, resulting in the enzymatic activation of MKP-3. |
==About this Structure== | ==About this Structure== | ||
| Line 14: | Line 14: | ||
[[Category: Single protein]] | [[Category: Single protein]] | ||
[[Category: Farooq, A.]] | [[Category: Farooq, A.]] | ||
| - | [[Category: Zhou, M | + | [[Category: Zhou, M M.]] |
[[Category: hydrolase]] | [[Category: hydrolase]] | ||
| - | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Feb 21 13:06:26 2008'' |
Revision as of 11:06, 21 February 2008
|
STRUCTURE OF ERK2 BINDING DOMAIN OF MAPK PHOSPHATASE MKP-3: STRUCTURAL INSIGHTS INTO MKP-3 ACTIVATION BY ERK2
Overview
MAP kinases (MAPKs), which control mitogenic signal transduction in all eukaryotic organisms, are inactivated by dual specificity MAPK phosphatases (MKPs). MKP-3, a prototypical MKP, achieves substrate specificity through its N-terminal domain binding to the MAPK ERK2, resulting in the activation of its C-terminal phosphatase domain. The solution structure and biochemical analysis of the ERK2 binding (EB) domain of MKP-3 show that regions that are essential for ERK2 binding partly overlap with its sites that interact with the C-terminal catalytic domain, and that these interactions are functionally coupled to the active site residues of MKP-3. Our findings suggest a novel mechanism by which the EB domain binding to ERK2 is transduced to cause a conformational change of the C-terminal catalytic domain, resulting in the enzymatic activation of MKP-3.
About this Structure
1HZM is a Single protein structure of sequence from Homo sapiens. Full crystallographic information is available from OCA.
Reference
Solution structure of ERK2 binding domain of MAPK phosphatase MKP-3: structural insights into MKP-3 activation by ERK2., Farooq A, Chaturvedi G, Mujtaba S, Plotnikova O, Zeng L, Dhalluin C, Ashton R, Zhou MM, Mol Cell. 2001 Feb;7(2):387-99. PMID:11239467
Page seeded by OCA on Thu Feb 21 13:06:26 2008