1ily
From Proteopedia
(New page: 200px<br /><applet load="1ily" size="450" color="white" frame="true" align="right" spinBox="true" caption="1ily" /> '''Solution Structure of Ribosomal Protein L18 ...) |
|||
| Line 1: | Line 1: | ||
| - | [[Image:1ily.gif|left|200px]]<br /><applet load="1ily" size=" | + | [[Image:1ily.gif|left|200px]]<br /><applet load="1ily" size="350" color="white" frame="true" align="right" spinBox="true" |
caption="1ily" /> | caption="1ily" /> | ||
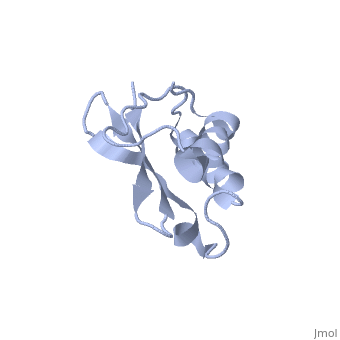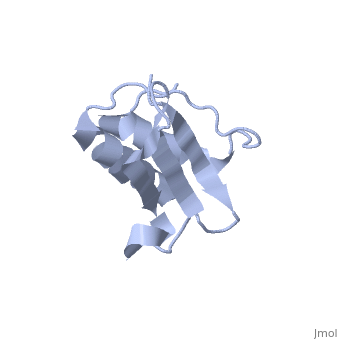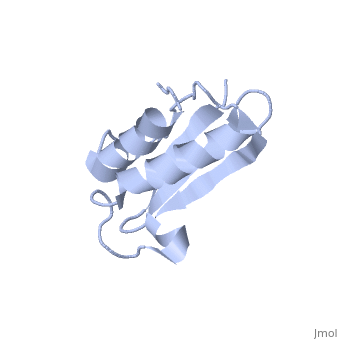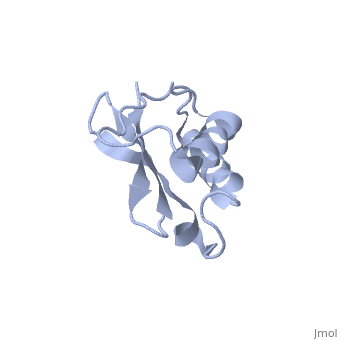
'''Solution Structure of Ribosomal Protein L18 of Thermus thermophilus'''<br /> | '''Solution Structure of Ribosomal Protein L18 of Thermus thermophilus'''<br /> | ||
==Overview== | ==Overview== | ||
| - | We have determined the solution structure of ribosomal protein L18 from | + | We have determined the solution structure of ribosomal protein L18 from Thermus thermophilus. L18 is a 12.5 kDa protein of the large subunit of the ribosome and binds to both 5 S and 23 S rRNA. In the uncomplexed state L18 folds to a mixed alpha/beta globular structure with a long disordered N-terminal region. We compared our high-resolution structure with RNA-complexed L18 from Haloarcula marismortui and T. thermophilus to examine RNA-induced as well as species-dependent structural differences. We also identified T. thermophilus S11 as a structural homologue and found that the structures of the RNA-recognition sites are conserved. Important features, for instance a bulge in the RNA-contacting beta-sheet, are conserved in both proteins. We suggest that the L18 fold recognizes a specific RNA motif and that the resulting RNA-protein-recognition module is tolerant to variations in sequence. |
==About this Structure== | ==About this Structure== | ||
| - | 1ILY is a [http://en.wikipedia.org/wiki/Single_protein Single protein] structure of sequence from [http://en.wikipedia.org/wiki/Thermus_thermophilus Thermus thermophilus]. Full crystallographic information is available from [http:// | + | 1ILY is a [http://en.wikipedia.org/wiki/Single_protein Single protein] structure of sequence from [http://en.wikipedia.org/wiki/Thermus_thermophilus Thermus thermophilus]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1ILY OCA]. |
==Reference== | ==Reference== | ||
| Line 14: | Line 14: | ||
[[Category: Thermus thermophilus]] | [[Category: Thermus thermophilus]] | ||
[[Category: Berglund, H.]] | [[Category: Berglund, H.]] | ||
| - | [[Category: Garber, M | + | [[Category: Garber, M B.]] |
| - | [[Category: Gongadze, G | + | [[Category: Gongadze, G M.]] |
[[Category: Hard, T.]] | [[Category: Hard, T.]] | ||
| - | [[Category: Rak, A | + | [[Category: Rak, A V.]] |
| - | [[Category: Shcherbakov, D | + | [[Category: Shcherbakov, D V.]] |
| - | [[Category: Woestenenk, E | + | [[Category: Woestenenk, E A.]] |
[[Category: mixed alpha/beta]] | [[Category: mixed alpha/beta]] | ||
| - | ''Page seeded by [http:// | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Feb 21 13:13:10 2008'' |
Revision as of 11:13, 21 February 2008
|
Solution Structure of Ribosomal Protein L18 of Thermus thermophilus
Overview
We have determined the solution structure of ribosomal protein L18 from Thermus thermophilus. L18 is a 12.5 kDa protein of the large subunit of the ribosome and binds to both 5 S and 23 S rRNA. In the uncomplexed state L18 folds to a mixed alpha/beta globular structure with a long disordered N-terminal region. We compared our high-resolution structure with RNA-complexed L18 from Haloarcula marismortui and T. thermophilus to examine RNA-induced as well as species-dependent structural differences. We also identified T. thermophilus S11 as a structural homologue and found that the structures of the RNA-recognition sites are conserved. Important features, for instance a bulge in the RNA-contacting beta-sheet, are conserved in both proteins. We suggest that the L18 fold recognizes a specific RNA motif and that the resulting RNA-protein-recognition module is tolerant to variations in sequence.
About this Structure
1ILY is a Single protein structure of sequence from Thermus thermophilus. Full crystallographic information is available from OCA.
Reference
The solution structure of ribosomal protein L18 from Thermus thermophilus reveals a conserved RNA-binding fold., Woestenenk EA, Gongadze GM, Shcherbakov DV, Rak AV, Garber MB, Hard T, Berglund H, Biochem J. 2002 May 1;363(Pt 3):553-61. PMID:11964156
Page seeded by OCA on Thu Feb 21 13:13:10 2008