We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
2j5k
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
==About this Structure== | ==About this Structure== | ||
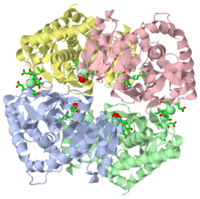
| - | [[2j5k]] is a 4 chain structure | + | [[2j5k]] is a 4 chain structure with sequence from [http://en.wikipedia.org/wiki/Haloarcula_marismortui Haloarcula marismortui]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2J5K OCA]. |
==See Also== | ==See Also== | ||
| Line 15: | Line 15: | ||
==Reference== | ==Reference== | ||
| - | <ref group="xtra">PMID:017211074</ref><ref group="xtra">PMID:012581646 | + | <ref group="xtra">PMID:017211074</ref><ref group="xtra">PMID:012581646</ref><ref group="xtra">PMID:017812611</ref><references group="xtra"/> |
[[Category: Haloarcula marismortui]] | [[Category: Haloarcula marismortui]] | ||
[[Category: Malate dehydrogenase]] | [[Category: Malate dehydrogenase]] | ||
Revision as of 19:34, 20 October 2012
| |||||||||
| 2j5k, resolution 2.00Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | |||||||||
| Activity: | Malate dehydrogenase, with EC number 1.1.1.37 | ||||||||
| Related: | 1d3a, 1gt2, 1hlp, 1o6z, 2hlp, 2j5q, 2j5r | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Contents |
2.0 A RESOLUTION STRUCTURE OF THE WILD TYPE MALATE DEHYDROGENASE FROM HALOARCULA MARISMORTUI (RADIATION DAMAGE SERIES)
Template:ABSTRACT PUBMED 17211074
About this Structure
2j5k is a 4 chain structure with sequence from Haloarcula marismortui. Full crystallographic information is available from OCA.
See Also
Reference
- Fioravanti E, Vellieux FM, Amara P, Madern D, Weik M. Specific radiation damage to acidic residues and its relation to their chemical and structural environment. J Synchrotron Radiat. 2007 Jan;14(Pt 1):84-91. Epub 2006 Dec 15. PMID:17211074 doi:10.1107/S0909049506038623
- Irimia A, Ebel C, Madern D, Richard SB, Cosenza LW, Zaccai G, Vellieux FM. The Oligomeric states of Haloarcula marismortui malate dehydrogenase are modulated by solvent components as shown by crystallographic and biochemical studies. J Mol Biol. 2003 Feb 21;326(3):859-73. PMID:12581646
- Dym O, Mevarech M, Sussman JL. Structural Features That Stabilize Halophilic Malate Dehydrogenase from an Archaebacterium. Science. 1995 Mar 3;267(5202):1344-1346. PMID:17812611 doi:267/5202/1344