We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Fragment-Based Drug Discovery
From Proteopedia
(Difference between revisions)
| Line 60: | Line 60: | ||
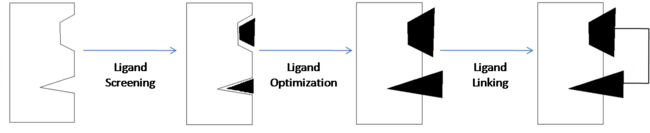
==== Ligand Linking ==== | ==== Ligand Linking ==== | ||
| - | |||
| - | |||
| - | |||
| - | Applying these 3-D structures to the drug design process involves using either structure-based drug design (SBDD) or ligand-based drug design (LBDD). | ||
| - | |||
| - | ==== ABT-737 ==== | ||
| - | |||
| - | One example of drug discovery using SAR by NMR includes the development of <scene name='Sandbox_reserved_394/Abt-737/1'>ABT-737</scene>. This compound has been shown to effectively inhibit the over-expression of <scene name='Sandbox_reserved_394/Bcl-xl/1'>Bcl-xl</scene> which is a protein that is commonly observed to be over-expressed in many types of cancers. It acts an inhibitor of apoptosis and may also contribute to chemotherapy resistance. Bcl-xl inhibition by ABT-737 therefore, allows apoptosis to occur and helps to prevent chemo-resistance. | ||
| - | |||
| - | ===== How SAR by NMR was used to develop ABT-737 ===== | ||
| - | |||
| - | Three ligands with moderate affinity for Bcl-xl were analyzed using SAR by NMR in order to develop ABT-737. The structural components that allow the ligands to bind to the protein were then linked together to form ABT-737 - the final compound with high-affinity for Bcl-xl. | ||
| - | |||
| - | <scene name='Sandbox_reserved_394/Compound_1/2'>Compound 1</scene> is a 4'-fluoro-biphenyl-4-carboxylic acid. SAR by NMR was used to identify the interactions that this compound forms with Bcl-xl. The fluorobiphenyl system is hydrophobic and its interactions form a <scene name='Sandbox_reserved_394/Compound_1/4'>"hydrophobic pocket"</scene> around the fluorobiphenyl system. The <scene name='Sandbox_reserved_394/Compound_1/5'>carboxyilic acid portion binds near Gly 142</scene> of Bcl-xl. The carboxylic acid is later substituted with an acyl sulfonamide (shown in compound 2) which provides increased affinity. | ||
| - | |||
| - | <scene name='Sandbox_reserved_394/Compound_2/1'>Compound 2</scene> binds with high affinity to Bcl-xl. The <scene name='Sandbox_reserved_394/Compound_2/2'>acylsulfonamide portion forms a hydrogen bond with Gly 142</scene>. The substitution of this sulfonamide for the carboxylic acid from compound 1 allows compound 2 to form a much stronger bond with Bcl-xl by bringing the shared acidic proton in closer proximity to GLY 142. The | ||
| - | |||
| - | |||
| - | |||
| - | Once the components responsible for binding are identified, they can be modified, as in the case of compound 1 where the carboxylic acid was substituted with an acyl sulfonamide, and then they are linked together to create a compound with optimal binding affinity. | ||
Revision as of 16:23, 31 October 2012
Drug Design: Fragment-Based Drug Discovery
| |||||||||||
References
- ↑ Oltersdorf T., Elmore S. W., Shoemaker A. R. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Vol 435|2 June 2005|doi:10.1038/nature03579
- ↑ Shuker S. B., Hajduk P. J., Meadows R. P., Fesik S. W. Discovering High-Affinity Ligands for Proteins: SAR by NMR. Science; Nov 29, 1996; 274, 5292; ProQuest Central pg. 1531.
- ↑ Pandit D. LIGAND-BASED DRUG DESIGN: I. CONFORMATIONAL STUDIES OF GBR 12909 ANALOGS AS COCAINE ANTAGONISTS; II. 3D-QSAR STUDIES OF SALVINORIN A ANALOGS AS εΑΡΡΑ OPIOID AGONISTS. http://archives.njit.edu/vol01/etd/2000s/2007/njit-etd2007-051/njit-etd2007-051.pdf