We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Fragment-Based Drug Discovery
From Proteopedia
(Difference between revisions)
| Line 26: | Line 26: | ||
===== ABT-737: ligand screening ===== | ===== ABT-737: ligand screening ===== | ||
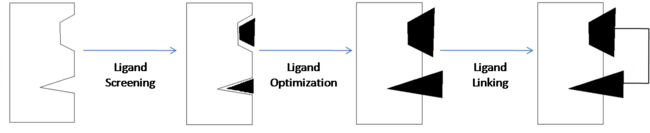
| - | <scene name='Sandbox_reserved_394/Compound_1/7'>Two fragments</scene> were found to have moderate affinity for Bcl-xl. <scene name='Sandbox_reserved_394/Compound_1/9'>Compound 1</scene> is a fluorobiphenylcarboxylic acid. It occupies <scene name='Sandbox_reserved_394/Binding_site_1/2'>binding site 1</scene> of Bcl-xl. The fluorobiphenyl | + | <scene name='Sandbox_reserved_394/Compound_1/7'>Two fragments</scene> were found to have moderate affinity for Bcl-xl. <scene name='Sandbox_reserved_394/Compound_1/9'>Compound 1</scene> is a fluorobiphenylcarboxylic acid. It occupies <scene name='Sandbox_reserved_394/Binding_site_1/2'>binding site 1</scene> of Bcl-xl which consists of Phe 101, Tyr 105, Ala 108, Phe 109, Leu 136, Gly 142, Arg 143, and Ala 146. The fluorobiphenyl system of compound 1 is very hydrophobic and therefore, these residues form a <scene name='Sandbox_reserved_394/Compound_1/4'>"hydrophobic pocket"</scene> around the system. The <scene name='Sandbox_reserved_394/Compound_1/5'>carboxyilic acid portion of compound 1 binds near Gly 142</scene> of Bcl-xl. |
| - | <scene name='Sandbox_reserved_394/Compound_1/3'>Compound 2</scene> is a napthalene-based alcohol which occupies <scene name='Sandbox_reserved_394/Binding_site_2/ | + | <scene name='Sandbox_reserved_394/Compound_1/3'>Compound 2</scene> is a napthalene-based alcohol which occupies <scene name='Sandbox_reserved_394/Binding_site_2/4'>binding site 2</scene>. This binding site includes Ala 97, Glu 100, Phe 101, Val 145, and Tyr 199. This particular fragment also is involved with hydrophobic interactions with Bcl-xl, although they are not as strong as in the case of compound 1. |
==== Ligand Optimization ==== | ==== Ligand Optimization ==== | ||
| Line 41: | Line 41: | ||
===== ABT-737: ligand optimization ===== | ===== ABT-737: ligand optimization ===== | ||
| - | Compounds 1 & 2 exhibited very poor binding affinity for Bcl-xl. The optimization of these two compounds resulted in <scene name='Sandbox_reserved_394/Compound_2/1'>Compound 3</scene>. In order to improve the binding affinity, the carboxylic acid of compound 1 was substituted with an acyl sulfonamide to capitalize on the hydrophilic interaction with the protein. This <scene name='Sandbox_reserved_394/Compound_2/ | + | Compounds 1 & 2 exhibited very poor binding affinity for Bcl-xl. The optimization of these two compounds resulted in <scene name='Sandbox_reserved_394/Compound_2/1'>Compound 3</scene>. In order to improve the binding affinity, the carboxylic acid of compound 1 was substituted with an acyl sulfonamide to capitalize on the hydrophilic interaction with the protein. This <scene name='Sandbox_reserved_394/Compound_2/3'>acylsulfonamide portion forms a hydrogen bond with Gly 142</scene> thereby increasing the affinity for Bcl-xl. The substitution of the sulfonamide actually allows the acidic proton to get closer to Gly 142 than it could in the carboxylic acid, which is why it is able to bind stronger to the amino acid. |
| - | Compound 2 was important in identifying the hydrophobicity of binding site 2 but was substituted with a <scene name='Sandbox_reserved_394/Nitro_thio_phenyl_sub/1'>3-nitro-4-(2-phenylthioethyl)aminophenyl group</scene>. This substitution more efficiently binds to site 2 through | + | Compound 2 was important in identifying the hydrophobicity of binding site 2 but was substituted with a <scene name='Sandbox_reserved_394/Nitro_thio_phenyl_sub/1'>3-nitro-4-(2-phenylthioethyl)aminophenyl group</scene>. This substitution more efficiently binds to site 2 through <scene name='Sandbox_reserved_394/Pi_stacking/2'>п stacking with Phe 101 and Tyr 199</scene>. This idea of using a known ligand to develop another ligand, and eventually drugs, is known as ligand-based drug design. |
{| class="wikitable collapsible collapsed" | {| class="wikitable collapsible collapsed" | ||
Revision as of 02:28, 7 November 2012
Drug Design: Fragment-Based Drug Discovery
| |||||||||||
References
- ↑ 1.0 1.1 Shuker S. B., Hajduk P. J., Meadows R. P., Fesik S. W. Discovering High-Affinity Ligands for Proteins: SAR by NMR. Science; Nov 29, 1996; 274, 5292; ProQuest Central pg. 1531.
- ↑ Oltersdorf T., Elmore S. W., Shoemaker A. R. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Vol 435|2 June 2005|doi:10.1038/nature03579
- ↑ Pandit D. LIGAND-BASED DRUG DESIGN: I. CONFORMATIONAL STUDIES OF GBR 12909 ANALOGS AS COCAINE ANTAGONISTS; II. 3D-QSAR STUDIES OF SALVINORIN A ANALOGS AS εΑΡΡΑ OPIOID AGONISTS. http://archives.njit.edu/vol01/etd/2000s/2007/njit-etd2007-051/njit-etd2007-051.pdf