We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Grb10 SH2 Domain
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
==Dimerization of the Grb10 SH2 Domain== | ==Dimerization of the Grb10 SH2 Domain== | ||
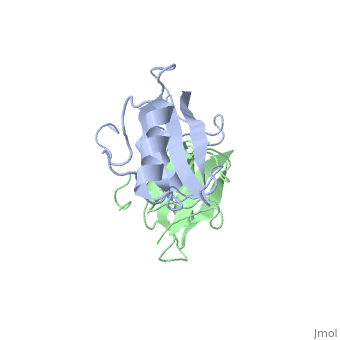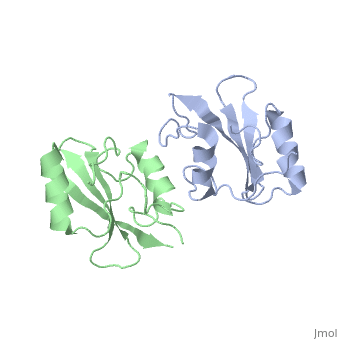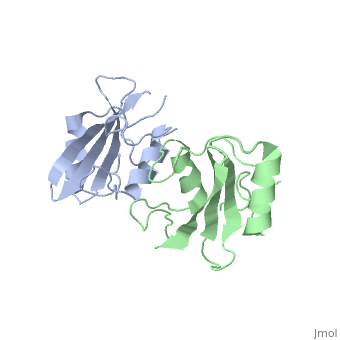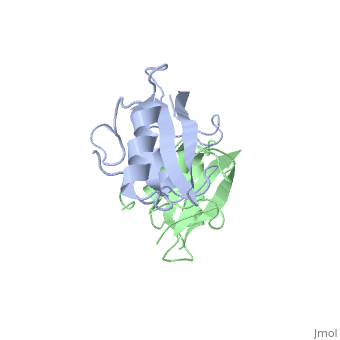
| - | The crystal structure of Grb10 SH2 domain was an important step to understanding how this protein interacts with IGF1 receptors, and although the SH2 domain functions as an independent segment, it forms a dimer in physiological environments. The <scene name='Grb10_SH2_Domain/Dimer_interface/ | + | The crystal structure of Grb10 SH2 domain was an important step to understanding how this protein interacts with IGF1 receptors, and although the SH2 domain functions as an independent segment, it forms a dimer in physiological environments. The <scene name='Grb10_SH2_Domain/Dimer_interface/2'>dimer interface</scene> exists due to the intermolecular forces between Phenylalanine515, Tyrosine516, and Asparganine519 of each independent monomer. The structure of Grb10 SH2 forms similar SH2 domains found in other proteins, which have an <scene name='Grb10_SH2_Domain/Alpha_helix/1'>alpha helix</scene> on the outsides with anti-parallel <scene name='Grb10_SH2_Domain/Beta_sheet/1'>beta sheets</scene>. |
<scene name='Grb10_SH2_Domain/Dimer_interface/2'>Zoom?</scene> | <scene name='Grb10_SH2_Domain/Dimer_interface/2'>Zoom?</scene> | ||
Revision as of 09:04, 7 November 2012
| |||||||||||
Interaction Between Grb10 and E3 Ubiquitin Ligase NEDD4
WOOOOOOOO
|
Grb10 Gene Inhibition Affects Body Composition, and Insulin Signaling
References
- ↑ Stein EG, Ghirlando R, Hubbard SR. Structural basis for dimerization of the Grb10 Src homology 2 domain. Implications for ligand specificity. J Biol Chem. 2003 Apr 11;278(15):13257-64. Epub 2003 Jan 27. PMID:12551896 doi:http://dx.doi.org/10.1074/jbc.M212026200