1pvo
From Proteopedia
(New page: 200px<br /><applet load="1pvo" size="450" color="white" frame="true" align="right" spinBox="true" caption="1pvo, resolution 3.00Å" /> '''X-ray crystal struct...) |
|||
| Line 1: | Line 1: | ||
| - | [[Image:1pvo.gif|left|200px]]<br /><applet load="1pvo" size=" | + | [[Image:1pvo.gif|left|200px]]<br /><applet load="1pvo" size="350" color="white" frame="true" align="right" spinBox="true" |
caption="1pvo, resolution 3.00Å" /> | caption="1pvo, resolution 3.00Å" /> | ||
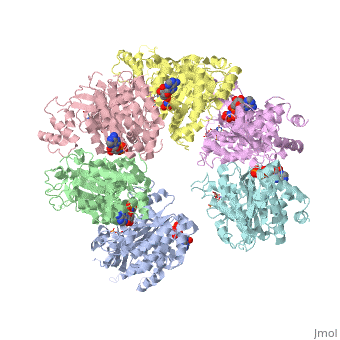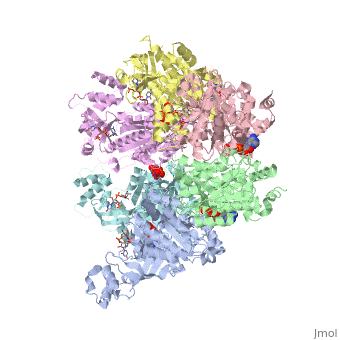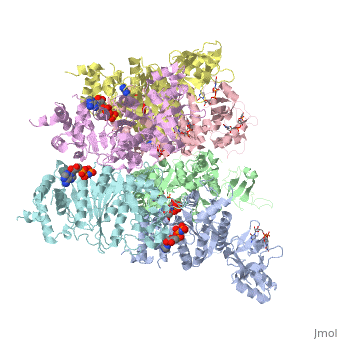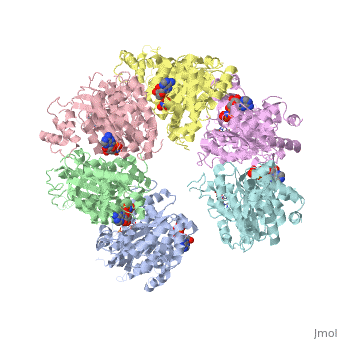
'''X-ray crystal structure of Rho transcription termination factor in complex with ssRNA substrate and ANPPNP'''<br /> | '''X-ray crystal structure of Rho transcription termination factor in complex with ssRNA substrate and ANPPNP'''<br /> | ||
==Overview== | ==Overview== | ||
| - | In bacteria, one of the major transcriptional termination mechanisms | + | In bacteria, one of the major transcriptional termination mechanisms requires a RNA/DNA helicase known as the Rho factor. We have determined two structures of Rho complexed with nucleic acid recognition site mimics in both free and nucleotide bound states to 3.0 A resolution. Both structures show that Rho forms a hexameric ring in which two RNA binding sites--a primary one responsible for target mRNA recognition and a secondary one required for mRNA translocation and unwinding--point toward the center of the ring. Rather than forming a closed ring, the Rho hexamer is split open, resembling a "lock washer" in its global architecture. The distance between subunits at the opening is sufficiently wide (12 A) to accommodate single-stranded RNA. This open configuration most likely resembles a state poised to load onto mRNA and suggests how related ring-shaped enzymes may be breached to bind nucleic acids. |
==About this Structure== | ==About this Structure== | ||
| - | 1PVO is a [http://en.wikipedia.org/wiki/Single_protein Single protein] structure of sequence from [http://en.wikipedia.org/wiki/Escherichia_coli Escherichia coli] with ANP as [http://en.wikipedia.org/wiki/ligand ligand]. Full crystallographic information is available from [http:// | + | 1PVO is a [http://en.wikipedia.org/wiki/Single_protein Single protein] structure of sequence from [http://en.wikipedia.org/wiki/Escherichia_coli Escherichia coli] with <scene name='pdbligand=ANP:'>ANP</scene> as [http://en.wikipedia.org/wiki/ligand ligand]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1PVO OCA]. |
==Reference== | ==Reference== | ||
| Line 13: | Line 13: | ||
[[Category: Escherichia coli]] | [[Category: Escherichia coli]] | ||
[[Category: Single protein]] | [[Category: Single protein]] | ||
| - | [[Category: Berger, J | + | [[Category: Berger, J M.]] |
[[Category: Skordalakes, E.]] | [[Category: Skordalakes, E.]] | ||
[[Category: ANP]] | [[Category: ANP]] | ||
[[Category: rho-anppnp-ssrna complex]] | [[Category: rho-anppnp-ssrna complex]] | ||
| - | ''Page seeded by [http:// | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Feb 21 14:32:59 2008'' |
Revision as of 12:33, 21 February 2008
|
X-ray crystal structure of Rho transcription termination factor in complex with ssRNA substrate and ANPPNP
Overview
In bacteria, one of the major transcriptional termination mechanisms requires a RNA/DNA helicase known as the Rho factor. We have determined two structures of Rho complexed with nucleic acid recognition site mimics in both free and nucleotide bound states to 3.0 A resolution. Both structures show that Rho forms a hexameric ring in which two RNA binding sites--a primary one responsible for target mRNA recognition and a secondary one required for mRNA translocation and unwinding--point toward the center of the ring. Rather than forming a closed ring, the Rho hexamer is split open, resembling a "lock washer" in its global architecture. The distance between subunits at the opening is sufficiently wide (12 A) to accommodate single-stranded RNA. This open configuration most likely resembles a state poised to load onto mRNA and suggests how related ring-shaped enzymes may be breached to bind nucleic acids.
About this Structure
1PVO is a Single protein structure of sequence from Escherichia coli with as ligand. Full crystallographic information is available from OCA.
Reference
Structure of the Rho transcription terminator: mechanism of mRNA recognition and helicase loading., Skordalakes E, Berger JM, Cell. 2003 Jul 11;114(1):135-46. PMID:12859904
Page seeded by OCA on Thu Feb 21 14:32:59 2008