Herceptin - Mechanism of Action
From Proteopedia
| Line 28: | Line 28: | ||
*<scene name='Sandbox_reserved_Herceptin/Sub-domain_iv/2'>sub-domain IV</scene> | *<scene name='Sandbox_reserved_Herceptin/Sub-domain_iv/2'>sub-domain IV</scene> | ||
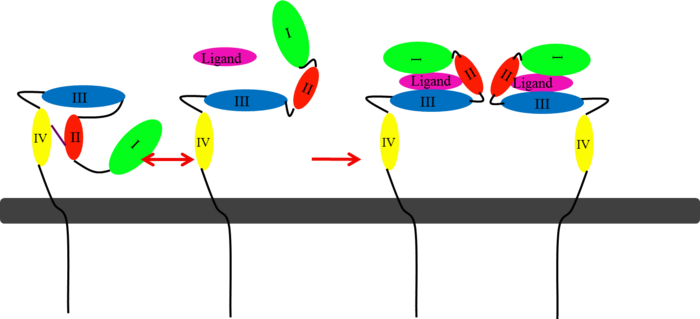
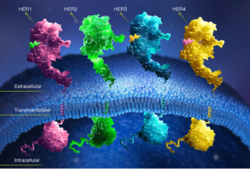
| - | In EGFR, HER3, and HER4 sub-domains I and III remain in a “closed” conformation until a ligand binds. Sub-domain II remains hidden making contact with sub-domain IV creating a "tether" (See figure ). The tether between sub-domain II and IV can be temporarily broken in order for the receptor to become more available for a ligand to bind. Although unsure, evidence suggests that the ligand binds to sub-domain I and/or sub-domain III; also, there’s some evidence of ligand-binding to sub-domain IV. Once ligand-binding is initiated, the receptor experiences a conformational change allowing sub-domains I and III to become | + | In EGFR, HER3, and HER4 sub-domains I and III remain in a “closed” conformation until a ligand binds. Sub-domain II remains hidden making contact with sub-domain IV creating a "tether" (See figure ). The tether between sub-domain II and IV can be temporarily broken in order for the receptor to become more available for a ligand to bind. Although unsure, evidence suggests that the ligand binds to sub-domain I and/or sub-domain III; also, there’s some evidence of ligand-binding to sub-domain IV. Once ligand-binding is initiated, the receptor experiences a conformational change allowing sub-domains I and III to become closer together resulting in the “open” conformation. This open conformation exposes sub-domain II which allows for dimerization between receptors. Although some evidence suggests that sub-domain IV is necessary for ligand-binding, stabilizing the extracellular domain, and locking the receptor in an open conformation, the exact function is still unknown. Once the dimerization between the two receptors takes place, a cross-phosphorylation reaction between the two tyrosine kinases occurs following the activation of certain cell signaling pathways. These pathways include: |
*mitogen-activated protein kinase (MAPK) | *mitogen-activated protein kinase (MAPK) | ||
*phosphoinositide 3-kinase (PI3K/Akt) | *phosphoinositide 3-kinase (PI3K/Akt) | ||
| Line 44: | Line 44: | ||
=== Herceptin === | === Herceptin === | ||
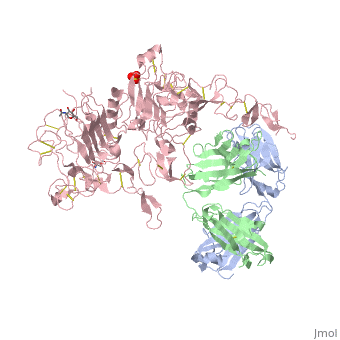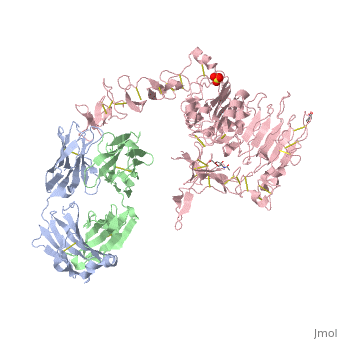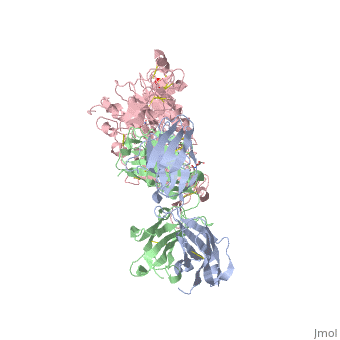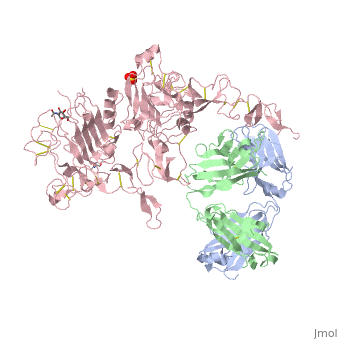
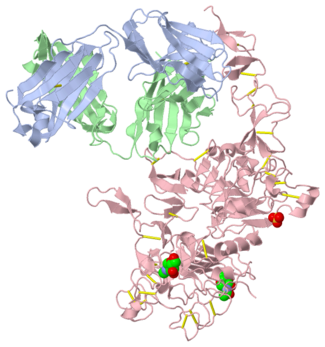
| - | <scene name='Sandbox_reserved_Herceptin/Herceptin/1'>Herceptin</scene> is a monoclonal antibody proven to be a clinically effective treatment in fighting HER2+ breast cancer. There is also some clinical evidence suggesting that Herceptin enhances the effects of chemotherapy. Herceptin binds to the juxtamembrane region of HER2 on the C-terminal portion of sub-domain IV. The interaction formed by Herceptin and HER2 is mediated by three regions on HER2 that form three loops: residues 557-561 (loop 1), 570-573 (loop 2), and 593-603 (loop 3). Loops 1 and 3 are formed primarily by electrostatic interactions and loop 2 is formed by hydrophobic contacts. The key amino acids involved in the interaction between HER2 and Herceptin can be seen <scene name='Sandbox_reserved_Herceptin/Interactions1/2'>here</scene>. Herceptin's key amino acids (green) include: Asn 30 and Thr 94 on the light chain and Tyr 33, Arg 50, Trp 99, Gly 103, and Tyr 105 on the heavy chain. Key amino acids involved in HER2 are as follows: loop 1 (blue) which includes Glu 558 and Asp 560, loop 2 ( | + | <scene name='Sandbox_reserved_Herceptin/Herceptin/1'>Herceptin</scene> is a monoclonal antibody proven to be a clinically effective treatment in fighting HER2+ breast cancer. There is also some clinical evidence suggesting that Herceptin enhances the effects of chemotherapy. Herceptin binds to the juxtamembrane region of HER2 on the C-terminal portion of sub-domain IV. The interaction formed by Herceptin and HER2 is mediated by three regions on HER2 that form three loops: residues 557-561 (loop 1), 570-573 (loop 2), and 593-603 (loop 3). Loops 1 and 3 are formed primarily by electrostatic interactions and loop 2 is formed by hydrophobic contacts. The key amino acids involved in the interaction between HER2 and Herceptin can be seen <scene name='Sandbox_reserved_Herceptin/Interactions1/2'>here</scene>. Herceptin's key amino acids (green) include: Asn 30 and Thr 94 on the light chain and Tyr 33, Arg 50, Trp 99, Gly 103, and Tyr 105 on the heavy chain. Key amino acids involved in HER2 are as follows: loop 1 (blue) which includes Glu 558 and Asp 560, loop 2 (red) which includes Asp 570 and Phe 573, and loop 3 (orange) which includes Gln 602. |
Herceptin acts by four different mechanisms of action: | Herceptin acts by four different mechanisms of action: | ||
| Line 57: | Line 57: | ||
An added significant mechanism of action of Herceptin is the prevention of the formation of p95HER2. By binding to the juxtamembrane region Herceptin sterically hinders any cleavage of the site ultimately preventing the formation of the highly active p95HER2. | An added significant mechanism of action of Herceptin is the prevention of the formation of p95HER2. By binding to the juxtamembrane region Herceptin sterically hinders any cleavage of the site ultimately preventing the formation of the highly active p95HER2. | ||
| - | The next two mechanisms of action for Herceptin involve intracellular inhibition. By the binding of Herceptin to the extracellular region of HER2 Herceptin is able to prevent downstream activation of cell signals that induce cell proliferation and angiogenesis. By repression of the proangiogenic factors Herceptin is also able to induce antiangiogenic factors. | + | The next two mechanisms of action for Herceptin involve intracellular inhibition. By the binding of Herceptin to the extracellular region of HER2 Herceptin is able to prevent downstream activation of cell signals that induce cell proliferation and angiogenesis. By repression of the proangiogenic factors Herceptin is also able to induce antiangiogenic factors. Inhibiting these intracellular processes Herceptin prevents any further blood vessels from developing towards the tumor and prevents the tumor from growing any larger. |
One limitation with this therapy is that it does not prevent HER2 from dimerizing with HER3. This makes it possible for the activation of the PI3K pathway which increases the ability of the cell to survive. Further therapies targeting the prevention of HER2 to dimerize with other members of the HER family will be necessary for future investigational studies. | One limitation with this therapy is that it does not prevent HER2 from dimerizing with HER3. This makes it possible for the activation of the PI3K pathway which increases the ability of the cell to survive. Further therapies targeting the prevention of HER2 to dimerize with other members of the HER family will be necessary for future investigational studies. | ||
</StructureSection>[[Image:EGFRs Mechanism.png|700px|left|thumb| <span style="font-size:1.2em;">This picture illustrates the mechanism in which EGFR, HER3, and HER4 change conformation in order to dimerize and activate further cell signaling. A) Sub-domain I (green) is sepearated from sub-domain III (blue). Sub-domain II (red) forms an interaction (purple line) with sub-domain IV (yellow). This conformation allows sub-domain II to be hidden and unavailable for dimerization. B) The interaction between sub-domain II and sub-domain IV can be temporarily broken allowing for the receptor to become more available for ligand-binding. C) Upon ligand-binding, if the interaction of sub-domain II and IV is still formed, the interaction between sub-domain II and sub-domain IV is broken and allows sub-domain II to become available for homo- or hetero-dimerization with another receptor from the HER family.</span>]] | </StructureSection>[[Image:EGFRs Mechanism.png|700px|left|thumb| <span style="font-size:1.2em;">This picture illustrates the mechanism in which EGFR, HER3, and HER4 change conformation in order to dimerize and activate further cell signaling. A) Sub-domain I (green) is sepearated from sub-domain III (blue). Sub-domain II (red) forms an interaction (purple line) with sub-domain IV (yellow). This conformation allows sub-domain II to be hidden and unavailable for dimerization. B) The interaction between sub-domain II and sub-domain IV can be temporarily broken allowing for the receptor to become more available for ligand-binding. C) Upon ligand-binding, if the interaction of sub-domain II and IV is still formed, the interaction between sub-domain II and sub-domain IV is broken and allows sub-domain II to become available for homo- or hetero-dimerization with another receptor from the HER family.</span>]] | ||
Revision as of 16:47, 8 November 2012
Basics
Introduction
Breast cancer is the most common type of cancer found among women. Although it is rarely seen in men, one in eight women will be diagnosed with breast cancer within their lifetime. Patients exhibiting an over-expression in Human Epidermal Growth Factor Receptor 2 (HER2) account for 25% of all breast cancer. HER2+ patients often experience a more aggressive cancer resulting in more metastasized tumors. The statistics show a poor prognosis for HER2+ patients with a 5-year survival rate of 68%. Herceptin (also known as trastuzumab) was approved by the FDA in September of 1998 for HER2+ patients and has been shown to be an effective tool in the battle against breast cancer.
HER2
HER2 is one of four human epidermal growth factor receptors (EGFR , HER2, HER3, and HER4). These receptors are part of a family of receptor tyrosine kinases responsible for cell proliferation and differentiation.These human epidermal growth factor receptors exist on the cell surface and, with the exception of HER2, bind to specific ligands (epidermal growth factors). Over 11 different ligands for the epidermal growth factor receptors have been identified. After binding with these ligands the HER family is able to homodimerize (with the exception of HER2) or heterodimerize with one another. This dimerization causes a cross-phosphorylation of the intracellular tyrosine kinases between the two receptors and ultimately activates a cell signaling pathway.
HER2 is the only receptor within this family that is constitutively active being able to dimerize with other HER family members acting in a ligand-independent manner. This continuous activation of the cell signal pathway causes an increase in cell division; thus, potentially causing a tumor.
Herceptin
Herceptin, generic trastuzumab, is a monoclonal antibody. Herceptin is an effective treatment for breast cancer for the reason that it binds to the extracellular domain of HER2 and, by multiple mechanisms of action, can prevent cell proliferation as well as target these HER2+ cells for destruction by the immune system.
| |||||||||||