Herceptin - Mechanism of Action
From Proteopedia
| Line 8: | Line 8: | ||
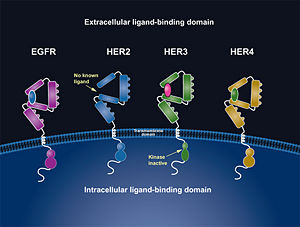
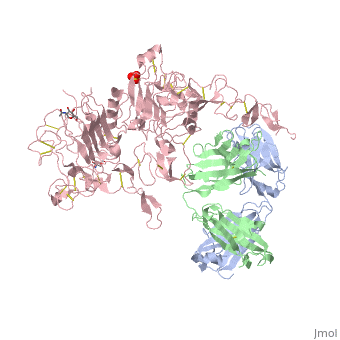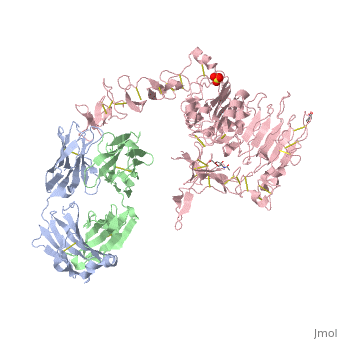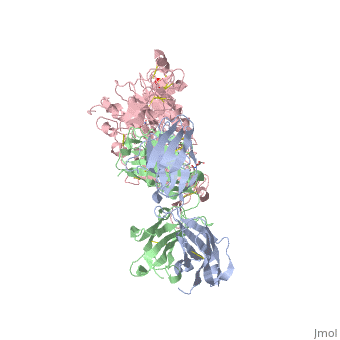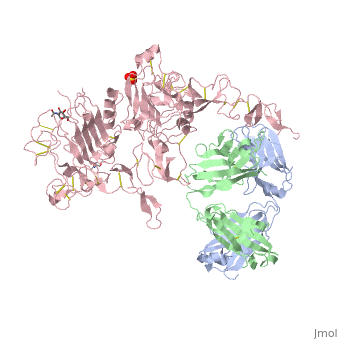
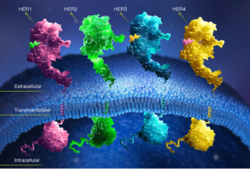
HER2 is one of four human epidermal growth factor receptors (EGFR , HER2, HER3, and HER4). These receptors are part of a family of receptor tyrosine kinases responsible for cell proliferation and differentiation. [[Image:New Resource 1.jpg|thumb|right|300 px|(Figure 2) HER family]] This family is known as the ErbB family, being that these proteins are encoded by the ERBB genes, and is also known as the HER family. The HER family are plasma membrane-bound and contain an extracellular ligand-binding domain, a transmembrane domain, and an intracellular domain. | HER2 is one of four human epidermal growth factor receptors (EGFR , HER2, HER3, and HER4). These receptors are part of a family of receptor tyrosine kinases responsible for cell proliferation and differentiation. [[Image:New Resource 1.jpg|thumb|right|300 px|(Figure 2) HER family]] This family is known as the ErbB family, being that these proteins are encoded by the ERBB genes, and is also known as the HER family. The HER family are plasma membrane-bound and contain an extracellular ligand-binding domain, a transmembrane domain, and an intracellular domain. | ||
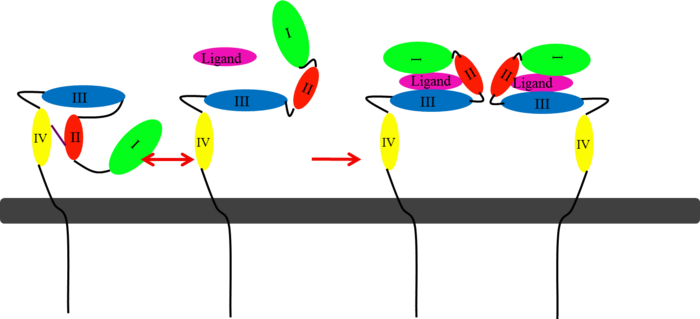
| - | These human epidermal growth factor receptors exist on the cell surface and, with the exception of HER2, bind to specific ligands (epidermal growth factors). Over 11 different ligands for the epidermal growth factor receptors have been identified. After binding with these ligands the HER family is able to homodimerize | + | These human epidermal growth factor receptors exist on the cell surface and, with the exception of HER2, bind to specific ligands (epidermal growth factors). Over 11 different ligands for the epidermal growth factor receptors have been identified. After binding with these ligands the HER family is able to homodimerize or heterodimerize with one another. This dimerization causes a cross-phosphorylation of the intracellular tyrosine kinases between the two receptors and ultimately activates a cell signaling pathway. |
HER2 is the only receptor within this family that is constitutively active being able to dimerize with other HER family members acting in a ligand-independent manner. This continuous activation of the cell signal pathway causes an increase in cell division; thus, potentially causing a tumor. | HER2 is the only receptor within this family that is constitutively active being able to dimerize with other HER family members acting in a ligand-independent manner. This continuous activation of the cell signal pathway causes an increase in cell division; thus, potentially causing a tumor. | ||
| Line 36: | Line 36: | ||
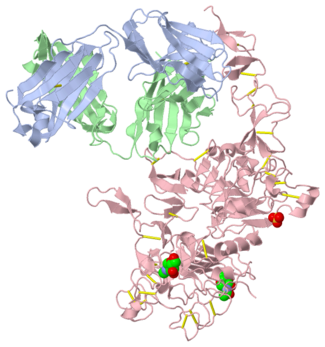
[[Image:EGFRs.png|thumb|left|250 px|(Figure 5)]] | [[Image:EGFRs.png|thumb|left|250 px|(Figure 5)]] | ||
| - | HER2 structure differs in that sub-domains I and III are in constant contact resulting in a permanent open conformation. Sub-domains I and III in HER2 are stabilized by core hydrophobic residues surrounded by hydrophilic contacts facilitating the stabilization of this interaction. This fixed conformation allows the receptor to act in a ligand-independent manner and permits the access of sub-domain II for dimerization. Perhaps this may explain why a high-affinity ligand for HER2 has not yet been recognized. The absence of sub-domain II and IV contact can be explained by the mutations of Gly 563 and His 565 found in the other members of the HER family to Pro and Phe respectively. Since sub-domain II is always available for dimerization it is free to form heterodimers with any of the other epidermal growth factor receptors; it does not form a homodimer. Asp 285 is the only residue in sub-domain II not conserved between EGFR and HER2. Leu replaces Asp 285 in HER2 and can explain why HER2 | + | HER2 structure differs in that sub-domains I and III are in constant contact resulting in a permanent open conformation. Sub-domains I and III in HER2 are stabilized by core hydrophobic residues surrounded by hydrophilic contacts facilitating the stabilization of this interaction. This fixed conformation allows the receptor to act in a ligand-independent manner and permits the access of sub-domain II for dimerization. Perhaps this may explain why a high-affinity ligand for HER2 has not yet been recognized. The absence of sub-domain II and IV contact can be explained by the mutations of Gly 563 and His 565 found in the other members of the HER family to Pro and Phe respectively. Since sub-domain II is always available for dimerization it is free to form heterodimers with any of the other epidermal growth factor receptors; it does not form a homodimer. Asp 285 is the only residue in sub-domain II not conserved between EGFR and HER2. Leu replaces Asp 285 in HER2 and can explain why HER2 forms homodimers to a lesser extent. Once dimerized, HER2 activates the MAPK pathway, which in turn initiates cell proliferation, migration, differentiation, and angiogenesis. |
HER2 also has another potentially harmful ability. The juxtamembrane region of the extracellular domain is prone to proteolytic cleavage resulting in a protein called p95HER2. <scene name='Sandbox_reserved_Herceptin/Truncated/1'>p95HER2</scene> is the truncated form of HER2 and has been shown to have an increased ability to cross-phosphorylate with the other receptors of the HER family and has increased cellular signaling capabilities. | HER2 also has another potentially harmful ability. The juxtamembrane region of the extracellular domain is prone to proteolytic cleavage resulting in a protein called p95HER2. <scene name='Sandbox_reserved_Herceptin/Truncated/1'>p95HER2</scene> is the truncated form of HER2 and has been shown to have an increased ability to cross-phosphorylate with the other receptors of the HER family and has increased cellular signaling capabilities. | ||
Revision as of 22:23, 9 November 2012
Basics
Introduction
Breast cancer is the most common type of cancer found among women. Although it is rarely seen in men, one in eight women will be diagnosed with breast cancer within their lifetime. Patients exhibiting an over-expression in Human Epidermal Growth Factor Receptor 2 (HER2) account for 25% of all breast cancer. HER2+ patients often experience a more aggressive cancer resulting in more metastasized tumors. The statistics show a poor prognosis for HER2+ patients with a 5-year survival rate of 68%. Herceptin (also known as trastuzumab) was approved by the FDA in September of 1998 for HER2+ patients and has been shown to be an effective tool in the battle against breast cancer.
HER2
HER2 is one of four human epidermal growth factor receptors (EGFR , HER2, HER3, and HER4). These receptors are part of a family of receptor tyrosine kinases responsible for cell proliferation and differentiation. This family is known as the ErbB family, being that these proteins are encoded by the ERBB genes, and is also known as the HER family. The HER family are plasma membrane-bound and contain an extracellular ligand-binding domain, a transmembrane domain, and an intracellular domain.These human epidermal growth factor receptors exist on the cell surface and, with the exception of HER2, bind to specific ligands (epidermal growth factors). Over 11 different ligands for the epidermal growth factor receptors have been identified. After binding with these ligands the HER family is able to homodimerize or heterodimerize with one another. This dimerization causes a cross-phosphorylation of the intracellular tyrosine kinases between the two receptors and ultimately activates a cell signaling pathway.
HER2 is the only receptor within this family that is constitutively active being able to dimerize with other HER family members acting in a ligand-independent manner. This continuous activation of the cell signal pathway causes an increase in cell division; thus, potentially causing a tumor.
Herceptin
Herceptin, generic trastuzumab, is a monoclonal antibody. Herceptin is an effective treatment for breast cancer for the reason that it binds to the extracellular domain of HER2 and, by multiple mechanisms of action, can prevent cell proliferation as well as target these HER2+ cells for destruction by the immune system.
| |||||||||||