Sean Swale/Human Thrombin Inhibitor
From Proteopedia
| Line 1: | Line 1: | ||
== Human Thrombin Inhibitor == | == Human Thrombin Inhibitor == | ||
| - | Thrombin is a serine protease that cleaves fibrinogen to allow fibrin to form stringy networks that trap red blood cells to form clots. Thrombin is a serine protease because it cleaves fibrinogen with its serine residue. Thrombin when made by the body is tethered to blood vessels so that it cannot cause clots throughout the body causing strokes and heart attacks. Additionally, thrombin is only activated for a few seconds to limit the clotted area to the injured area<ref>January 2002 Molecule of the Month by David Goodsell | + | Thrombin is a serine protease that cleaves fibrinogen to allow fibrin to form stringy networks that trap red blood cells to form clots. Thrombin is a serine protease because it cleaves fibrinogen with its serine residue. Thrombin when made by the body is tethered to blood vessels so that it cannot cause clots throughout the body causing strokes and heart attacks. Additionally, thrombin is only activated for a few seconds to limit the clotted area to the injured area<ref>January 2002 Molecule of the Month by David Goodsell http://www.rcsb.org/pdb/101/motm.do?momID=25 </ref>. In cases such as dialysis a blood thinner like hirudin is needed to make sure a blood clot is not created in the body. Unfortunately, when hirudin was used it could be required up to four times per week which heavily taxes the body. Thus new thrombin inhibitors with greater specificity for the thrombin binding site were developed in vitro. <ref> DOI: 10.1002/cmdc.201200292</ref> |
| - | |||
==Introduction== | ==Introduction== | ||
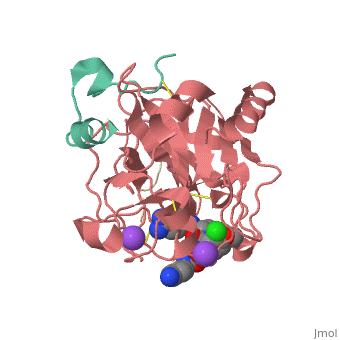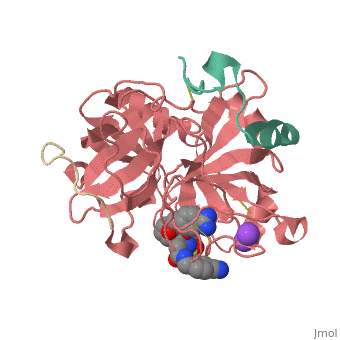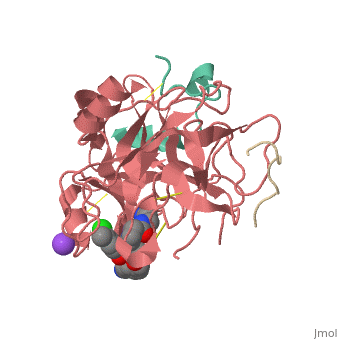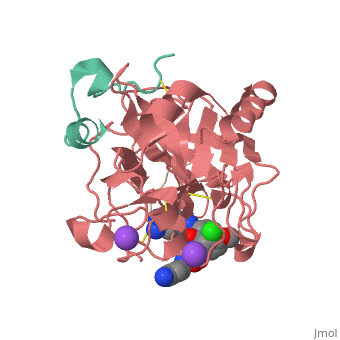
<Structure load='3utu' size='500' frame='false' align='right' |caption='Human Thrombin' scene='Insert optional scene name here' StructureSection> | <Structure load='3utu' size='500' frame='false' align='right' |caption='Human Thrombin' scene='Insert optional scene name here' StructureSection> | ||
| Line 13: | Line 12: | ||
The | The | ||
(p1, p2) <ref> M. R. Wiley, N. Y. Chirgadze, D. K. Clawson, T. J. Craft, D. S. GiffordMoore, N. D. Jones, J. L. Olkowski, L. C. Weir, G. F. Smith, Bioorg. Med. Chem. Lett. 1996, 6, 2387. http://www.sciencedirect.com/science/article/pii/0960894X96004428 </ref> | (p1, p2) <ref> M. R. Wiley, N. Y. Chirgadze, D. K. Clawson, T. J. Craft, D. S. GiffordMoore, N. D. Jones, J. L. Olkowski, L. C. Weir, G. F. Smith, Bioorg. Med. Chem. Lett. 1996, 6, 2387. http://www.sciencedirect.com/science/article/pii/0960894X96004428 </ref> | ||
| + | |||
==References== | ==References== | ||
{{reflist}} | {{reflist}} | ||
Revision as of 23:48, 13 November 2012
Human Thrombin Inhibitor
Thrombin is a serine protease that cleaves fibrinogen to allow fibrin to form stringy networks that trap red blood cells to form clots. Thrombin is a serine protease because it cleaves fibrinogen with its serine residue. Thrombin when made by the body is tethered to blood vessels so that it cannot cause clots throughout the body causing strokes and heart attacks. Additionally, thrombin is only activated for a few seconds to limit the clotted area to the injured area[1]. In cases such as dialysis a blood thinner like hirudin is needed to make sure a blood clot is not created in the body. Unfortunately, when hirudin was used it could be required up to four times per week which heavily taxes the body. Thus new thrombin inhibitors with greater specificity for the thrombin binding site were developed in vitro. [2]
Introduction
| |||||||||||