Sandbox Reserved 390
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
==INTRODUCTION == | ==INTRODUCTION == | ||
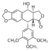
| - | The research team described the structural basis by which an anticancer drug etoposide kills cancer cells by interacting with its cellular targets human DNA topoisomerase type II<ref>PMID: 21778401</ref>. In the close-up stereo representation of the <scene name='Sandbox_Reserved_390/Top/8'>etoposide-binding site(s),</scene> cartoon-and-stick representation shows the insertion of two etoposide molecules into two cleavage sites [etoposide surrounded by orange mesh that represent active site (etoposide in red & grey representation), the DNA chain is in red and blue, and the magnesium is in green]. | + | The research team described the structural basis by which an anticancer drug etoposide kills cancer cells by interacting with its cellular targets human DNA topoisomerase type II<ref name="rasmol"><ref>PMID: 21778401</ref>. In the close-up stereo representation of the <scene name='Sandbox_Reserved_390/Top/8'>etoposide-binding site(s),</scene> cartoon-and-stick representation shows the insertion of two etoposide molecules into two cleavage sites [etoposide surrounded by orange mesh that represent active site (etoposide in red & grey representation), the DNA chain is in red and blue, and the magnesium is in green]. |
==ENZYME== | ==ENZYME== | ||
Type II topoisomerases (TOP2s) are abundant enzymes that play an essential role in <scene name='Sandbox_Reserved_390/Top/5'>DNA</scene> replication and transcription and are important targets for cancer chemotherapeutic drugs. These enzymes briefly cleave a pair of opposing phosphodiester bonds four base pairs apart, generating a TOP2-DNA cleavage complex. | Type II topoisomerases (TOP2s) are abundant enzymes that play an essential role in <scene name='Sandbox_Reserved_390/Top/5'>DNA</scene> replication and transcription and are important targets for cancer chemotherapeutic drugs. These enzymes briefly cleave a pair of opposing phosphodiester bonds four base pairs apart, generating a TOP2-DNA cleavage complex. | ||
| Line 14: | Line 14: | ||
TOP2’s DNA cleavage activity is usually referred to as a double-edged sword; failure to reseal the enzyme-mediated DNA break can lead to cell death. Several potent anticancer drugs, such as<scene name='Sandbox_Reserved_390/Top/7'> etoposide</scene>, doxorubicin and mitoxantrone, exploit this harmful aspect of TOP2 and promote the formation of cytotoxic DNA lesions by increasing the steady-state level of cleavage complexes. <ref> Kathryn L. Gilroy, Chrysoula Leontiou, Kay Padget, Jeremy H. Lakey and Caroline A. Austin* "mAMSA resistant human topoisomerase IIβ mutation G465D has reduced ATP hydrolysis activity” Oxford JournalsLife Sciences Nucleic Acids Research Volume 34, Issue 5Pp. 1597-1607. [http://nar.oxfordjournals.org/content/34/5/1597 DOI: 10.1093/nar/gkl057]</ref> | TOP2’s DNA cleavage activity is usually referred to as a double-edged sword; failure to reseal the enzyme-mediated DNA break can lead to cell death. Several potent anticancer drugs, such as<scene name='Sandbox_Reserved_390/Top/7'> etoposide</scene>, doxorubicin and mitoxantrone, exploit this harmful aspect of TOP2 and promote the formation of cytotoxic DNA lesions by increasing the steady-state level of cleavage complexes. <ref> Kathryn L. Gilroy, Chrysoula Leontiou, Kay Padget, Jeremy H. Lakey and Caroline A. Austin* "mAMSA resistant human topoisomerase IIβ mutation G465D has reduced ATP hydrolysis activity” Oxford JournalsLife Sciences Nucleic Acids Research Volume 34, Issue 5Pp. 1597-1607. [http://nar.oxfordjournals.org/content/34/5/1597 DOI: 10.1093/nar/gkl057]</ref> | ||
| - | In this paper, the researchers reported on the crystal structure of a large fragment of type II human topoisomerases β (hTOP2β core) complexed to DNA and to the anticancer drug etoposide to reveal structural details of drug-induced stabilization of a cleavage complex<ref | + | In this paper, the researchers reported on the crystal structure of a large fragment of type II human topoisomerases β (hTOP2β core) complexed to DNA and to the anticancer drug etoposide to reveal structural details of drug-induced stabilization of a cleavage complex<ref name="rasmol" />. This structure provided the first observation of a TOP2 ternary cleavage complex <scene name='Sandbox_Reserved_390/Top/9'>stabilized</scene> by an anticancer drug. |
The high-resolution structure of the hTOP2βcore-DNA-etoposide ternary complex reveals the intricate interplays between <scene name='Sandbox_Reserved_390/Top/6'>protein</scene>, <scene name='Sandbox_Reserved_390/Top/16'>DNA</scene> and <scene name='Sandbox_Reserved_390/Top/17'>drugs</scene>. This aspect is extremely important because all vertebrates possess two highly similar yet functionally distinct TOP2 isoforms. The α-isoform is particularly important for DNA replication and is usually present at high levels in fast growing cancer cells, whereas the β-isoform is mainly involved in transcription related processes. Although the inhibition of both TOP2 isoforms contributes to the drug-induced death of cancer cells, targeting of the β-isoform has been implicated in deleterious therapy related secondary malignancies. Therefore, it is desirable to develop the isoform specific TOP2-targeting agents. | The high-resolution structure of the hTOP2βcore-DNA-etoposide ternary complex reveals the intricate interplays between <scene name='Sandbox_Reserved_390/Top/6'>protein</scene>, <scene name='Sandbox_Reserved_390/Top/16'>DNA</scene> and <scene name='Sandbox_Reserved_390/Top/17'>drugs</scene>. This aspect is extremely important because all vertebrates possess two highly similar yet functionally distinct TOP2 isoforms. The α-isoform is particularly important for DNA replication and is usually present at high levels in fast growing cancer cells, whereas the β-isoform is mainly involved in transcription related processes. Although the inhibition of both TOP2 isoforms contributes to the drug-induced death of cancer cells, targeting of the β-isoform has been implicated in deleterious therapy related secondary malignancies. Therefore, it is desirable to develop the isoform specific TOP2-targeting agents. | ||
Revision as of 00:42, 14 November 2012
Human topoisomerase IIbeta in complex with DNA and etoposide
| |||||||||||
References
- ↑ 1.0 1.1 <ref>PMID: 21778401</li> <li id="cite_note-1">[[#cite_ref-1|↑]] Kathryn L. Gilroy, Chrysoula Leontiou, Kay Padget, Jeremy H. Lakey and Caroline A. Austin* "mAMSA resistant human topoisomerase IIβ mutation G465D has reduced ATP hydrolysis activity” Oxford JournalsLife Sciences Nucleic Acids Research Volume 34, Issue 5Pp. 1597-1607. [http://nar.oxfordjournals.org/content/34/5/1597 DOI: 10.1093/nar/gkl057]</li></ol></ref>