We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sean Swale/Human Thrombin Inhibitor
From Proteopedia
(Difference between revisions)
| Line 16: | Line 16: | ||
[[Image:Inhibitor 65.png|center|400px]] | [[Image:Inhibitor 65.png|center|400px]] | ||
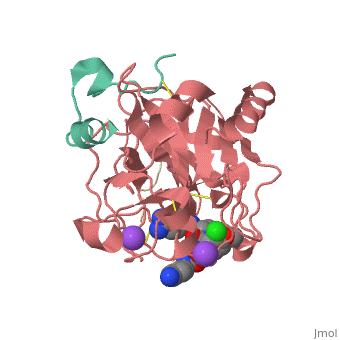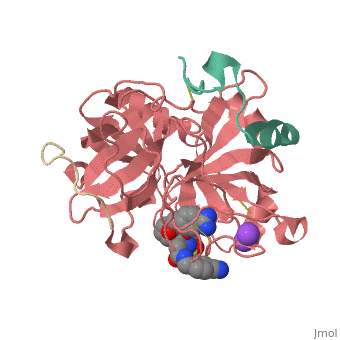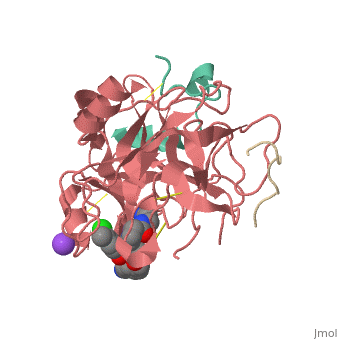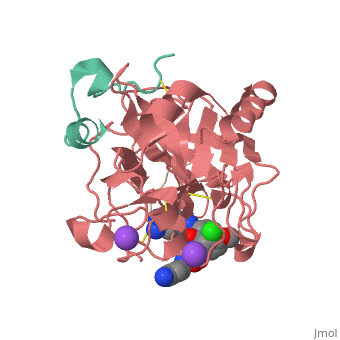
Inhibitor 65 additionally has a P4 sulfonyl group with a methoxy group at the end of the residue. It sits in tight VanderWaals structure with the surrounding residues Leu 99, Tyr 60A, Arg 97, Glu 97, and Asn 98.Additionally, the chlorine points to the carbonyl oxygen of Asn 98 and there is a weak Cl-π bond with Trp 215. For the P3 residue, there the backbone of asparagine forms and anti parallel beta sheet with Gly 216. The alkylated asparagine’s NH binds to water which then connects to Glu 192, and the NH of Gly 219 and Gly 216. | Inhibitor 65 additionally has a P4 sulfonyl group with a methoxy group at the end of the residue. It sits in tight VanderWaals structure with the surrounding residues Leu 99, Tyr 60A, Arg 97, Glu 97, and Asn 98.Additionally, the chlorine points to the carbonyl oxygen of Asn 98 and there is a weak Cl-π bond with Trp 215. For the P3 residue, there the backbone of asparagine forms and anti parallel beta sheet with Gly 216. The alkylated asparagine’s NH binds to water which then connects to Glu 192, and the NH of Gly 219 and Gly 216. | ||
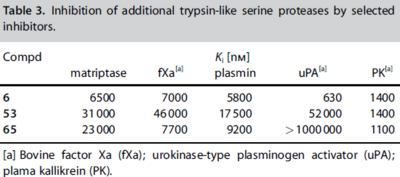
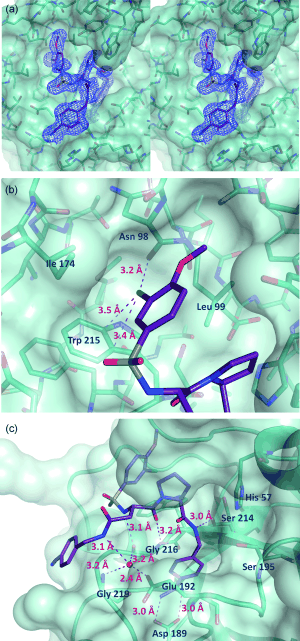
| - | [[Image:Thrombin1.gif| | + | [[Image:Thrombin1.gif|left|frame|300px|a) Stereo view of the complete inhibitor structure provided with its electron density in the active site of thrombin. Thrombin is shown with its solvent- accessible surface. b) The P4 sulfonyl residue is located in the aryl binding pocket. The chlorine atom points to the carbonyl oxygen of Asn 98 and accommodates a position above the indole moiety of Trp 215. The methoxy group perfectly fills the upper niche of the S3/4 pocket. c) The P3 side chain is directed into the solvent, where its amide NH binds via a bridging water molecule to Glu 192, Gly 216 and Gly 219.]] |
</StructureSection> | </StructureSection> | ||
Revision as of 21:18, 15 November 2012
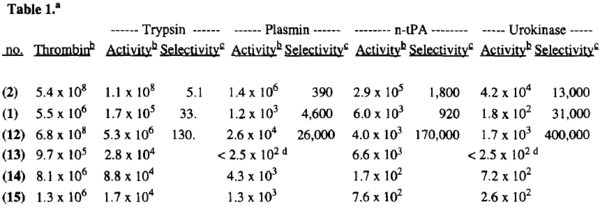
| |||||||||||
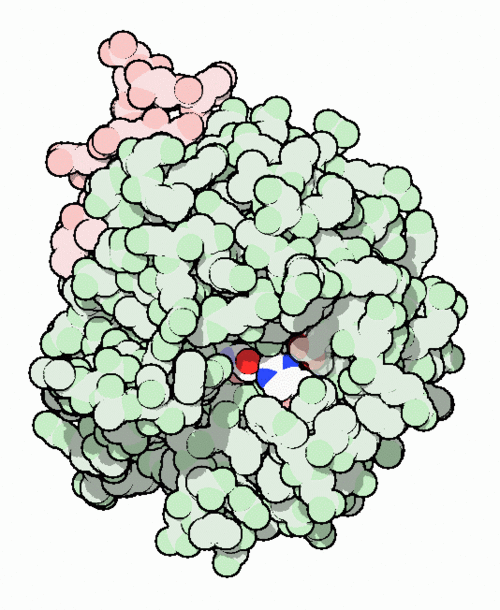
This is the of thrombin
References
- ↑ January 2002 Molecule of the Month by David Goodsell http://www.rcsb.org/pdb/101/motm.do?momID=25
- ↑ Steinmetzer T, Baum B, Biela A, Klebe G, Nowak G, Bucha E. Beyond Heparinization: Design of Highly Potent Thrombin Inhibitors Suitable for Surface Coupling. ChemMedChem. 2012 Aug 20. doi: 10.1002/cmdc.201200292. PMID:22907907 doi:10.1002/cmdc.201200292
- ↑ http://voices.yahoo.com/what-fibrinolytic-enzymes-6186058.html?cat=68
- ↑ M. R. Wiley, N. Y. Chirgadze, D. K. Clawson, T. J. Craft, D. S. GiffordMoore, N. D. Jones, J. L. Olkowski, L. C. Weir, G. F. Smith, Bioorg. Med. Chem. Lett. 1996, 6, 2387. http://www.sciencedirect.com/science/article/pii/0960894X96004428