Sean Swale/Human Thrombin Inhibitor
From Proteopedia
(Difference between revisions)
| Line 15: | Line 15: | ||
===P3 and P4 of Inhibitor 65=== | ===P3 and P4 of Inhibitor 65=== | ||
[[Image:Inhibitor 65.png|center|400px|frame|<ref> DOI: 10.1002/cmdc.201200292</ref> ]] | [[Image:Inhibitor 65.png|center|400px|frame|<ref> DOI: 10.1002/cmdc.201200292</ref> ]] | ||
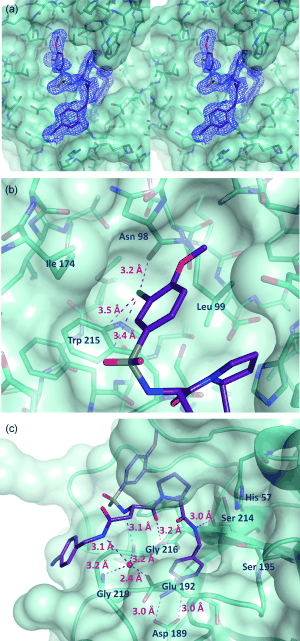
| - | Inhibitor 65 additionally has a <scene name='Sean_Swale/Human_Thrombin_Inhibitor/P4/1'>P4</scene> sulfonyl group with a methoxy group at the end of the residue. It sits in tight VanderWaals structure with the surrounding residues Leu 99, Tyr 60A, Arg 97, Glu 97, and Asn 98.Additionally, the chlorine points to the carbonyl oxygen of Asn 98 and there is a weak Cl-π bond with Trp 215. For the <scene name='Sean_Swale/Human_Thrombin_Inhibitor/P3_binding_site/6'>residue</scene>, there the backbone of asparagine forms and anti parallel beta sheet with Gly 216. The alkylated asparagine’s NH binds to water which then connects to Glu 192, and the | + | Inhibitor 65 additionally has a <scene name='Sean_Swale/Human_Thrombin_Inhibitor/P4/1'>P4</scene> sulfonyl group with a methoxy group at the end of the residue. The residue sits in a hydrophobic <scene name='Sean_Swale/Human_Thrombin_Inhibitor/P4_binding_pocket/1'>pocket</scene>. It sits in tight VanderWaals structure with the surrounding residues Leu 99, Tyr 60A, Arg 97, Glu 97, and Asn 98.The <scene name='Sean_Swale/Human_Thrombin_Inhibitor/P4_sulfonyl/1'>sulfonyl</scene> group holds its own VanderWaal forces. Additionally, the chlorine points to the carbonyl oxygen of Asn 98 and there is a weak Cl-π bond with Trp 215. For the <scene name='Sean_Swale/Human_Thrombin_Inhibitor/P3_binding_site/6'>residue</scene>, there the backbone of asparagine forms and anti parallel beta sheet with Gly 216. The alkylated asparagine’s NH binds to a water molecule which then connects to Glu 192, and the hydrogens of Gly 219 and Gly 216. |
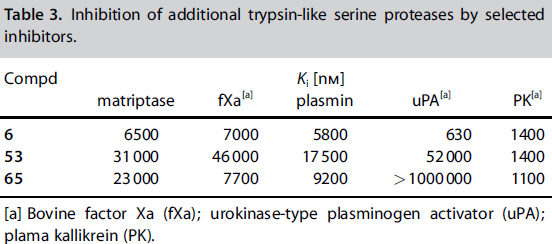
[[Image:Thrombin1.gif|left|frame|300px|a) Stereo view of the complete inhibitor structure provided with its electron density in the active site of thrombin. Thrombin is shown with its solvent- accessible surface. b) The P4 sulfonyl residue is located in the aryl binding pocket. The chlorine atom points to the carbonyl oxygen of Asn 98 and accommodates a position above the indole moiety of Trp 215. The methoxy group perfectly fills the upper niche of the S3/4 pocket. c) The P3 side chain is directed into the solvent, where its amide NH binds via a bridging water molecule to Glu 192, Gly 216 and Gly 219.<ref> DOI: 10.1002/cmdc.201200292</ref> ]] | [[Image:Thrombin1.gif|left|frame|300px|a) Stereo view of the complete inhibitor structure provided with its electron density in the active site of thrombin. Thrombin is shown with its solvent- accessible surface. b) The P4 sulfonyl residue is located in the aryl binding pocket. The chlorine atom points to the carbonyl oxygen of Asn 98 and accommodates a position above the indole moiety of Trp 215. The methoxy group perfectly fills the upper niche of the S3/4 pocket. c) The P3 side chain is directed into the solvent, where its amide NH binds via a bridging water molecule to Glu 192, Gly 216 and Gly 219.<ref> DOI: 10.1002/cmdc.201200292</ref> ]] | ||
Revision as of 23:49, 15 November 2012
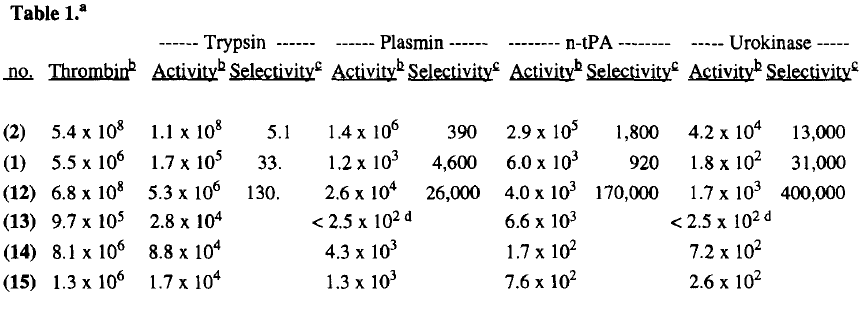
| |||||||||||
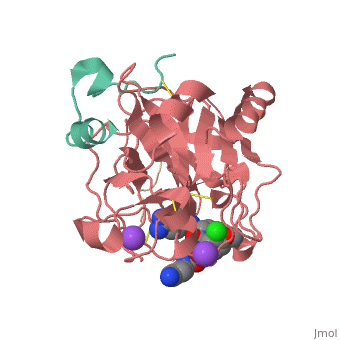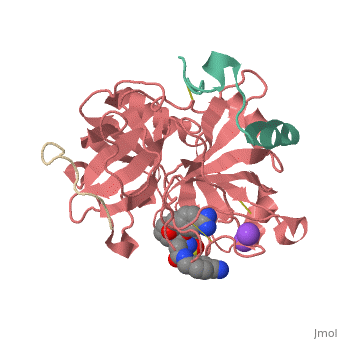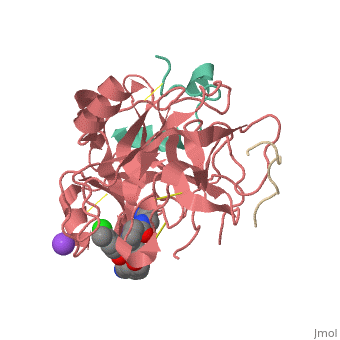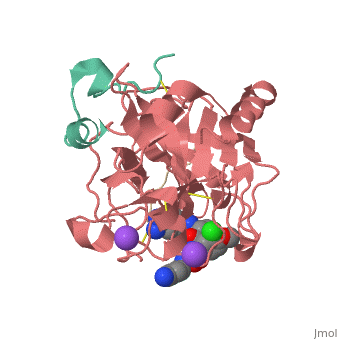
This is the of thrombin
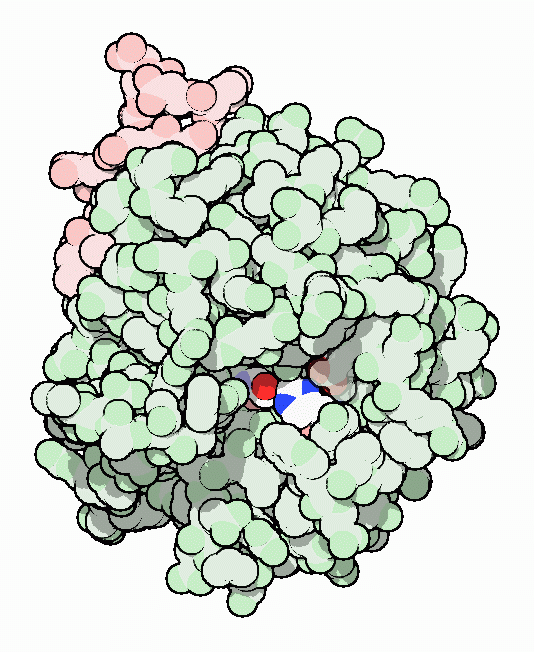
Thrombin with serine residue visible.[8]
References
- ↑ January 2002 Molecule of the Month by David Goodsell http://www.rcsb.org/pdb/101/motm.do?momID=25
- ↑ Steinmetzer T, Baum B, Biela A, Klebe G, Nowak G, Bucha E. Beyond Heparinization: Design of Highly Potent Thrombin Inhibitors Suitable for Surface Coupling. ChemMedChem. 2012 Aug 20. doi: 10.1002/cmdc.201200292. PMID:22907907 doi:10.1002/cmdc.201200292
- ↑ http://voices.yahoo.com/what-fibrinolytic-enzymes-6186058.html?cat=68
- ↑ M. R. Wiley, N. Y. Chirgadze, D. K. Clawson, T. J. Craft, D. S. GiffordMoore, N. D. Jones, J. L. Olkowski, L. C. Weir, G. F. Smith, Bioorg. Med. Chem. Lett. 1996, 6, 2387. http://www.sciencedirect.com/science/article/pii/0960894X96004428
- ↑ M. R. Wiley, N. Y. Chirgadze, D. K. Clawson, T. J. Craft, D. S. GiffordMoore, N. D. Jones, J. L. Olkowski, L. C. Weir, G. F. Smith, Bioorg. Med. Chem. Lett. 1996, 6, 2387. http://www.sciencedirect.com/science/article/pii/0960894X96004428
- ↑ Steinmetzer T, Baum B, Biela A, Klebe G, Nowak G, Bucha E. Beyond Heparinization: Design of Highly Potent Thrombin Inhibitors Suitable for Surface Coupling. ChemMedChem. 2012 Aug 20. doi: 10.1002/cmdc.201200292. PMID:22907907 doi:10.1002/cmdc.201200292
- ↑ Steinmetzer T, Baum B, Biela A, Klebe G, Nowak G, Bucha E. Beyond Heparinization: Design of Highly Potent Thrombin Inhibitors Suitable for Surface Coupling. ChemMedChem. 2012 Aug 20. doi: 10.1002/cmdc.201200292. PMID:22907907 doi:10.1002/cmdc.201200292
- ↑ January 2002 Molecule of the Month by David Goodsell http://www.rcsb.org/pdb/101/motm.do?momID=25