Sandbox Reserved 654
From Proteopedia
(→'''Mechanism''') |
(→'''Mechanism''') |
||
| Line 31: | Line 31: | ||
[[Image:F1.medium.gif |thumb|250 px|right| Acetylation of the lysine and its effects on chromatin remodeling..]] | [[Image:F1.medium.gif |thumb|250 px|right| Acetylation of the lysine and its effects on chromatin remodeling..]] | ||
The mechanism of protein-protein interaction for the bromodomain of PCAF with histone proteins begins with the acetylation of lysine residues on the histone tail. The acetylation causes a conformational change in the histones, which allows for transcriptional machinery to access DNA. The bromodomains of PCAF within a pretranscriptional initiation complex (PIC) bind to the acetyl-lysine of the histone to stabilize the complex so that transcription may begin. The bromodomains of PCAF have three major points of contact that allow for site-specific histone recognition. First, the acetylated lysine of the target protein enters a hydrophobic pocket embedded between the ZA and BC loops at the bottom of the protein. The Asn803 residue in the bromodomain forms a hydrogen bond with the amide nitrogen of the acetyl-lysine. Next, residues in the ZA and/or BC loops interact with residues adjacent to the acetyl-lysine, which reinforces the acetyl-lysine binding in the bromodomain. Finally, additional residues in the ZA and BC loops that face opposite to the bromodomain form hydrophobic and/ or electrostatic interaction with the target protein 3 residues away from the acetyl-lysine. This residue clamps on the BC loop together with the acetyl-lysine side chain that is bound inside the hydrophobic pocket of the bromodomain.[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3339198/] | The mechanism of protein-protein interaction for the bromodomain of PCAF with histone proteins begins with the acetylation of lysine residues on the histone tail. The acetylation causes a conformational change in the histones, which allows for transcriptional machinery to access DNA. The bromodomains of PCAF within a pretranscriptional initiation complex (PIC) bind to the acetyl-lysine of the histone to stabilize the complex so that transcription may begin. The bromodomains of PCAF have three major points of contact that allow for site-specific histone recognition. First, the acetylated lysine of the target protein enters a hydrophobic pocket embedded between the ZA and BC loops at the bottom of the protein. The Asn803 residue in the bromodomain forms a hydrogen bond with the amide nitrogen of the acetyl-lysine. Next, residues in the ZA and/or BC loops interact with residues adjacent to the acetyl-lysine, which reinforces the acetyl-lysine binding in the bromodomain. Finally, additional residues in the ZA and BC loops that face opposite to the bromodomain form hydrophobic and/ or electrostatic interaction with the target protein 3 residues away from the acetyl-lysine. This residue clamps on the BC loop together with the acetyl-lysine side chain that is bound inside the hydrophobic pocket of the bromodomain.[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3339198/] | ||
| + | |||
The histone acetyltransferase portion of PCAF helps with the transactivation of HIV-1 by acetylating Lys28 of Tat. The acetylated Lys28 of Tat interacts with positive elongation factors, which stimulates elongation of nascent HIV-1 transcripts. [http://www.sciencedirect.com/science/article/pii/S0969212602007542] | The histone acetyltransferase portion of PCAF helps with the transactivation of HIV-1 by acetylating Lys28 of Tat. The acetylated Lys28 of Tat interacts with positive elongation factors, which stimulates elongation of nascent HIV-1 transcripts. [http://www.sciencedirect.com/science/article/pii/S0969212602007542] | ||
| + | |||
| + | |||
| + | The bromodomain of PCAF recognizes acetylated Lys50 on Tat, but not Lys28. | ||
== '''Implications or Possible Applications''' == | == '''Implications or Possible Applications''' == | ||
Revision as of 00:42, 18 November 2012
| This Sandbox is Reserved from 30/08/2012, through 01/02/2013 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 636 through Sandbox Reserved 685. | |||||||
To get started:
More help: Help:Editing For more help, look at this link: http://proteopedia.org/w/Help:Getting_Started_in_Proteopedia
p300/CBP-associated factor
p300/CBP-associated factor (PCAF), [1] StructureStructure stuff[2] Catalytic Domain The of phenylalanine hydroxylase includes resides 143-410. This region has a basket-like arrangement consisting of 13 alpha-helices and 8 beta-strands. This region of the protein also includes the active site. The active site of PheOH can be found in the center of the catalytic domain and is characterized by a 13 Angstroms deep and 10 Angstroms wide hydrophobic pocket. Lining the active site are 3 glutamates, 2 histadines and 1 tyrosine residue along with hydrophobic residues for a total of 34 amino acids. Covering the entrance of the active site is a short loop consisting or residues 378-381. The center of each catalytic domain consists of an iron ion which is vital to the enzyme activity. The iron atom binds in the active site to . Histadine 285 and 290 were found to be required for the binding of iron through site directed mutagenisis studies. The iron ions are coordinated to three water molecules and arrange in an octahedral geometry. The active site also binds the . This cofactor binds closely to the iron ion and forms hydrogen bonds with two of the three water molecules. The cofactor also forms hydrogen bonds with the carbonyl oxygen of the protein residues including Ala322, Gly247, and Leu249 and the amide of Leu249.[3] Tetramerization Domain Phenylalanine Hydroxylase exists in equilibrium between a homodimer and a homotetramer. The region responsible for the tertamerization is the located at the C terminal end of the protein. It consists of residues 411-452. The tetramerization domain consists of 2 beta-strands forming a beta-ribbon and an alpha-helix that is 40 angstroms long. The four alpha helices, consisting of one from each monomer, pack into a coil coil motif with the helices arranged in an anti-parallel manner.[4] Regulatory Domain Housed in the N-terminus, the regulatory domain contains residues 19-142 and is more flexible than the other domains. The core of this domain contains an alpha beta sandwich and a beta alpha beta double motif. [5] MechanismThe mechanism of protein-protein interaction for the bromodomain of PCAF with histone proteins begins with the acetylation of lysine residues on the histone tail. The acetylation causes a conformational change in the histones, which allows for transcriptional machinery to access DNA. The bromodomains of PCAF within a pretranscriptional initiation complex (PIC) bind to the acetyl-lysine of the histone to stabilize the complex so that transcription may begin. The bromodomains of PCAF have three major points of contact that allow for site-specific histone recognition. First, the acetylated lysine of the target protein enters a hydrophobic pocket embedded between the ZA and BC loops at the bottom of the protein. The Asn803 residue in the bromodomain forms a hydrogen bond with the amide nitrogen of the acetyl-lysine. Next, residues in the ZA and/or BC loops interact with residues adjacent to the acetyl-lysine, which reinforces the acetyl-lysine binding in the bromodomain. Finally, additional residues in the ZA and BC loops that face opposite to the bromodomain form hydrophobic and/ or electrostatic interaction with the target protein 3 residues away from the acetyl-lysine. This residue clamps on the BC loop together with the acetyl-lysine side chain that is bound inside the hydrophobic pocket of the bromodomain.[8]
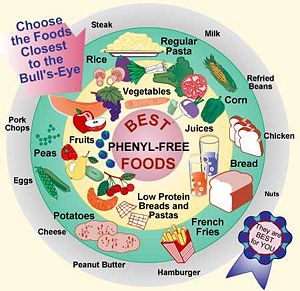
Implications or Possible ApplicationsThe first diagnosed cases of Phenylketonuria (PKU), otherwise known as Folling's Disease, were identified in 1934 by Norwegian doctor and biochemist Asbjorn Folling. Dr. Folling found that the urine of two of his young mentally handicapped patients contained a high level of phenylalanine. Follwing this discovery, it was found that the absence or malfunction of the phenylalanine hydroxylase enzyme is due to the mutation of the PAH gene and inherited autosomal recessively. This may result in a genetic disorder known as Phenylketonuria (PKU). This information was not utilized until the early 1950s when it was found that under a low phenylalanine diet, some of the symptoms found in children suffering from PKU could be reversed. Due to a diet rich in phenylalanine, this enzyme is vital in the regulation in phenylalanine plasma concentration by converting about 75% of the amino acid to tyrosine. Excessive amounts of phenylalanine has been shown to cause mental retardation in humans. Presently, it is regulation to screen newborns children for phenylketonuria with a simple blood or urine test. [6] Due to his discovery and development of the PKU test, Dr. Folling is remembered as one of the most important medical scientists that has not received a Nobel Prize for Physiology or Medicine. [7] SymptomsPhenylalanine plays a variety of roles in the body among which is the production of melanin, the pigment responsible for hair and skin color. Infants with an overabundance of this residue may therefore have a lighter skin, hair and eye color than those who do not. [8] Other symptoms may include: - Delayed mental and social skills - Head size significantly below normal - Hyperactivity - Jerking movements of the arms or legs - Mental retardation - Seizures - Skin rashes - Tremors - Unusual positioning of hands TreatmentTreatment for such a PKU is a low phenylalanine diet and early detection. Those who start the diet early and adhere to it will have better mental and physical health. Infants diagnosed with the disease can fed a specially made formula called Lofenalac while others should follow a diet plan as illustrated in the image to the left. The main rule to follow is to avoid protein sources rich in phenylalanine and sugars containing aspartame. Taking extra supplements like fish oil can replace the fatty acids missing from the phenylalanine free diet and may also improve neurological development. PKU can also be caused by a deficiency in or inability to regenerate tetrahydrobipternin, the cofactor essential to the function of PheOH. Although this is not usually the cause of PKU, patients can be treated by taking tetrahydrobiopterin supplements. If the diet is not strictly followed, mental retardation may result after the first year of life. [9]
References
|