The purpose of this page is to explain a semisynthetic derivative of podophyllotoxin
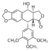
Chemical structure of podophyllotoxin
(etoposide) that demonstrates antitumor activity by inhibiting DNA topoisomerase II, thereby inhibiting DNA re-ligation which lead to apoptosis of the cancer cell and also, to describe the mechanisms of resistance of etoposide.
INTRODUCTION
The research team described the structural basis by which an anticancer drug etoposide kills cancer cells by interacting with its cellular targets human DNA topoisomerase type II[1]. In the close-up representation of the cartoon-and-stick representation shows the insertion of two etoposide molecules into two cleavage sites [etoposide surrounded by orange mesh that represent active site (etoposide in red & grey representation), the DNA chain is in red and blue, and the magnesium is in green]. The neighboring magnesium’s are believed to be use at both the ATP binding domain and in the cleavage core. In the nucleotide binding pocket magnesium is known to contact all of the phosphate groups and it’s possible that the changes in magnesium can be linked to a diminishing in the nucleotide-binding pocket.
ENZYME
Type II topoisomerases (TOP2s) are abundant enzymes that play an essential role in replication and transcription and are important targets for cancer chemotherapeutic drugs. These enzymes briefly cleave a pair of opposing phosphodiester bonds four base pairs apart, generating a TOP2-DNA cleavage complex.
TOP2’s DNA cleavage activity is usually referred to as a double-edged sword; failure to reseal the enzyme-mediated DNA break can lead to cell death. Several potent anticancer drugs, such as , and (all in green), exploit this harmful aspect of TOP2 and promote the formation of cytotoxic DNA lesions by increasing the stability level of cleavage complexes. [2]
In this paper, the researchers reported on the crystal structure of a large fragment of type II human topoisomerases β (hTOP2β core) complexed to DNA and to the anticancer drug etoposide to reveal structural details of drug-induced stabilization of a cleavage complex[3]. This structure provided the first observation of a TOP2 ternary cleavage complex by an anticancer drug.
The high-resolution structure of the hTOP2βcore-DNA-etoposide ternary complex reveals the intricate interplays between , and . This aspect is extremely important because all vertebrates possess two highly similar yet functionally distinct TOP2 isoforms. The α-isoform is particularly important for DNA replication and is usually present at high levels in fast growing cancer cells, whereas the β-isoform is mainly involved in transcription related processes. Although the inhibition of both TOP2 isoforms contributes to the drug-induced death of cancer cells, targeting of the β-isoform has been implicated in deleterious therapy related secondary malignancies. Therefore, it is desirable to develop the isoform specific TOP2-targeting agents.
COMPOUND ACTIVE SITE
This molecule has 36 represented with magenta helices and 40 represented with blue arrows in the . At the active site we can see how the (in green) is stabilized. The detail view of the show how the ligand is stabilized by amino acid (at position 503B in blue), (at position 479B in yellow with hydrogen bond interaction), (at position 778B in purple), also by nucleoside (in light blue at position 9F) and (in green at possition 13D showing a Pi-Pi interaction)
ETOPOSIDE RESISTANCE
The interplay between the protein, the DNA, and the drug explains the structure-activity relations of etoposide derivatives and the molecular basis of drug-resistant mutations. This resistance occurs via two mechanisms: 1) Decreased accumulation via increased P-glycoprotein; and 2) Changes in target proteins (mutation or decreased expression of topoisomerase II or decreased apoptosis due to mutation of p53).
1. Decreased accumulation via increased P-glycoprotein (a multidrug resistance): This drug resistance mechanism is characterized by decreased intracellular accumulation of drug facilitated by overexpression of the human multidrug resistance (mdrl) gene, causing overproduction of P-glycoprotein. This cell membrane protein acts as an export pump for a wide variety of unrelated foreign natural products. By maintaining lower intracellular levels of drug, lower drug concentration would be available to the target, which is topoisomerase II.
2. Changes in target proteins: This mechanism relates directly to the target enzyme; Either low enzyme levels or altered sensitivity of the enzyme for the drug confers resistance to that drug. This mechanism also confers a form of multidrug resistance; in that resistance to one topoisomerase II inhibitor through decreased or altered topoisomerase activity generally translates into resistance to most other topoisomerase II inhibitors.
Mutation of Glycine at position 465
According to earlier experiments, the glycine at position 465 in this structure is proposed to lie very close to the cleavage site and it’s is therefore expected to be involved in ATP hydrolysis. So the change of residue 465 from glycine to a more negatively charged (aspartic acid) is determined to create some localized hydrogen bonding at the backbone that can potentially alter the conformation of the enzyme (this change is determined to alter the charge interactions involved in binding site).
at position 465
at position 465

